-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2016; 6(1): 1-9
doi:10.5923/j.aac.20160601.01

Study on Activated from Melon (Citrullus lanatus) Husk as Natural Adsorbent for Removal of Hardness in Water
Mustapha S.1, Ndamitso M. M.1, Mohammed U. M.2, Adeosun N. O.3, Idris M.4
1Department of Chemistry, Federal University of Technology, Minna, Nigeria
2Department of Chemistry, Federal College of Education, Kontagora, Nigeria
3Department of Chemistry, Federal College of Education (Technical), Asaba, Nigeria
4Department of Primary Education Studies, Federal College of Education, Kontagora, Nigeria
Correspondence to: Mustapha S., Department of Chemistry, Federal University of Technology, Minna, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Batch adsorption process using activated melon (Citrullus lanatus) husk was studied to remove hardness (Ca2+ and Mg2+) from water sample. The adsorption of the metal ions was investigated by varying the adsorbent dose, contact time, solution pH and solution temperature. Experimental results showed that optimum times required for adsorption were 20 min for Ca2+ and 25 min for Mg2+. Also adsorption of the two metal ions increased with increasing adsorbent dosages. Thermodynamic parameters such as 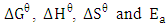 have been computed. The data showed the higher solution temperature values favoured the hardness removal by activated melon husk. It also revealed that the sorption process was spontaneous and exothermic in nature. The activation energy
have been computed. The data showed the higher solution temperature values favoured the hardness removal by activated melon husk. It also revealed that the sorption process was spontaneous and exothermic in nature. The activation energy 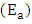 and sticking probability (S*) were used to assess physisorption mechanism between the adsorbate and adsorbent. The results showed that activated melon (Citrullus lanatus) husk is an alternative absorbent for the removal of Ca2+ and Mg2+ ions from hard water.
and sticking probability (S*) were used to assess physisorption mechanism between the adsorbate and adsorbent. The results showed that activated melon (Citrullus lanatus) husk is an alternative absorbent for the removal of Ca2+ and Mg2+ ions from hard water.
Keywords: Hardness, Thermodynamic, Sorption, Citrullus lanatus
Cite this paper: Mustapha S., Ndamitso M. M., Mohammed U. M., Adeosun N. O., Idris M., Study on Activated from Melon (Citrullus lanatus) Husk as Natural Adsorbent for Removal of Hardness in Water, Advances in Analytical Chemistry, Vol. 6 No. 1, 2016, pp. 1-9. doi: 10.5923/j.aac.20160601.01.
Article Outline
1. Introduction
- Wastes are arbitrarily disposed into the water bodies by industrial and household activities in some parts of the countries. The increase in water quality parameters such as heavy metals, nutrients and organic matter is as a result of the contamination of coastal and inland water bodies by generated wastes [1]. In some cases, water resources do not satisfy the desirable qualities regarding hardness and conductivity [2]. Calcium and magnesium are dominant divalent ions responsible for water hardness [3, 4] and problems of such water include scaling in boilers, washing machines and pipes [5], difficult lathering with soap, undesirable spots on sinks and clothes as well as toughening of skin and hair [6]. The determination of electrical conductivity provides a rapid and convenient means of estimating the concentration of electrolytes in water containing mostly mineral salts [7]. Calcium and magnesium play vital roles in the structure and functions of the human body. High intake of calcium and magnesium in drinking water could result in symptoms of toxicity such as a kidney stones [8], gastric and breast cancer, low blood pressure, muscle weakness, confusion and abnormal cardiac rhythm [9]. Therefore, the need to purify water which is not suitable for human consumption such as hard water cannot be overemphasized.Over the years, conventional methods such as electro- dialysis [10], ion-exchange [11], lime softening [12] and membrane filtration [13] have been studied for the removal of ionic species responsible for hardness. It is obvious that hard water treatment by these methods required high capital operations. Hence, finding inexpensive and efficiency developed processes remains a major concern. Adsorption process has become a promising approach for water treatment [14]. For the purpose of removing hardness ions from water, various adsorbent materials have been used such as Moringa oleifera [15, 16], Peanut hull [17], pumice [18] and Phyllanthus emblica [19].Melon (Citrullus lanatus) is a cucurbit crop that belongs to the family cucurbitaceae. Melon seeds ‘Egusi’ are grown, harvested and processed by removing the seeds from the fruits followed by washing and sun –drying. The flesh is inedible but the seeds are valuable in Africa. Melon husk is a lignocellulosic plant biomass and a waste product from the food industry that is discarded after shelling of melon seeds which litter the environment. The present study attempts to explore the potentials of adsorption by using this low-cost and locally available biomass in hardness and conductivity uptake. The results from this study will add knowledge to the field of water treatment and purification techniques which will in turn increase availability of safe and quality water to meet water demands of the people.
2. Materials and Methods
- Water sample obtained from a river in Kaduna metropolis, Nigeria was collected and parameters such as total solids, total dissolved solids, alkalinity, hardness, conductivity and turbidity was determined by standard methods described by [20] with slight modification.Total Hardness50 cm3 of sample was measured in an Erlenmeyer flask. 1 cm3 of ammonia buffer solution and 2 drops of Erichrome black-T indicator were added. The mixture was titrated with 0.01 M EDTA solution until the wine red colour of solution turns blue.
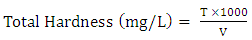 | (1) |
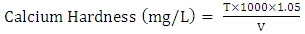 | (2) |
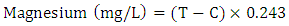 | (3) |
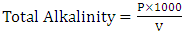 | (4) |
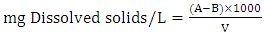 | (5) |
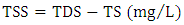 | (6) |
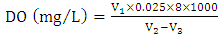 | (7) |
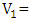 volume of tyrant (cm3);
volume of tyrant (cm3); 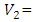 volume of sampling bottle after placing the stopper;
volume of sampling bottle after placing the stopper; 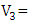 volume of manganous sulphate + potassium iodide solution (cm3).Electrical Conductivity (EC)The sample was measured using electrical conductivity meter at 25°C.
volume of manganous sulphate + potassium iodide solution (cm3).Electrical Conductivity (EC)The sample was measured using electrical conductivity meter at 25°C.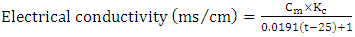 | (8) |
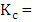 the cell constant,
the cell constant, 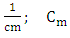 = measured conductance of the sample; t = observed temperature of the sample, °C.
= measured conductance of the sample; t = observed temperature of the sample, °C.2.1. Preparation of the Adsorbent
- Melon husk was washed several times with deionized water before use in order to remove impurities. The adsorbent was then treated with 0.5 M solution of H2SO4 for further purification. It was then washed with deionized water to remove the acid. The melon husk was then dried dehydrated at 55°C for 24 hours in an oven. The dried sample was milled and sieved to obtain 200 micrometer fraction. The sample was then carbonized in an electric furnace at 550°C for 15 min. The activated carbon produced was washed with deionized water and later oven dried before use.
2.2. Batch Experiments
- All experiments were conducted in batch modes in 250 cm3 conical flasks. Operating parameters including contact time, pH (2-8), temperature (30-70°C) and adsorbent mass (25-75 g/dm3) were investigated. Optimized adsorption times for the adsorbents were first examined by varying the contact time at room temperature, pH= 7.0 and adsorbent mass of 0.5 g per 0.04 dm3 of solution. The mixture was then shaken at 180 rpm at different predetermined time intervals and filtered. The percentage adsorption was determined as:
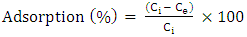 | (9) |
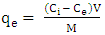 | (10) |
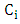 is initial concentration of metal uions in the solution (mgdm-3)
is initial concentration of metal uions in the solution (mgdm-3) 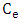 is the equilibrium concentration or initial concentration of metal ions in the solution (mgdm-3),
is the equilibrium concentration or initial concentration of metal ions in the solution (mgdm-3), 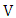 is the initial volume of metal ion solution used (dm3) and
is the initial volume of metal ion solution used (dm3) and 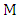 is the mass of adsorbent (g).
is the mass of adsorbent (g).2.3. Thermodynamic Studies
- Thermodynamic parameters were determined for temperatures ranging from 30 to 70°C using the equilibrium constant
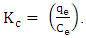 The standard change in free energy
The standard change in free energy 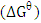 was determined as follows:
was determined as follows: 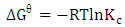 | (11) |
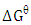 the standard free energy (kJ/mol), R is is the gas constant (8.314 J/molK) and T is temperature (K).
the standard free energy (kJ/mol), R is is the gas constant (8.314 J/molK) and T is temperature (K).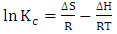 | (12) |
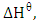 and standard entropy,
and standard entropy, 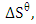 were calculated from the slope and the intercept of the linear plot of
were calculated from the slope and the intercept of the linear plot of 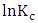 against
against 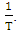
3. Results and Discussion
3.1. Physicochemical Properties of Water Sample
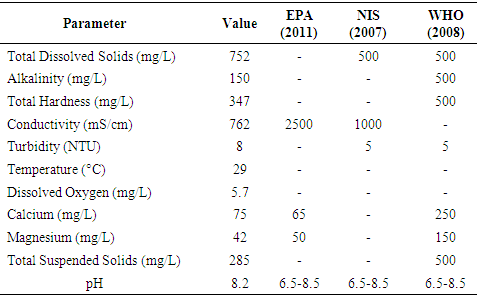
- Table 1, presents the physical parameters obtained from the analysis of the water sample from Kaduna urban area, Nigeria. Total dissolved solids (TDS) which measure the dissolved matter such as salts, organic matter and minerals in water were determined and the results show that the value of TDS was 752 mg/L. Nigeria Industrial Standard [20] recommended safe permissible limit for total dissolved solids in water is 500 mg/L. The water sample analyzed was considerably above the limit permitted by NIS [21]. Alkalinity aids in estimating the buffering capacity of wastewaters. The estimated value of alkalinity was found to be 150 mg/L. This finding does not corroborate the result reported by Rosal et al. [22], who reported alkalinity of 492 mg/L on effectiveness of wastewater treatment process. The low alkalinity value was due to the absence of dissolved limestone in the area. Hardness in water is due to carbonates, bicarbonates, sulphates and chlorides of Ca and Mg [23]. The recorded value for calcium was 75 mg/L respectively which is higher than standards of Environmental Protection Agency [24]. Water with a total hardness above 180 mg/L is termed hard [25]. Based on the information provided, water sample in this study can be classified as hard water. Turbidity was higher than the desirable limit of the World Health Organization 5.0 NTU. The increase in value could be associated with the mobilization of accumulated particles (organic and inorganic) from and within distribution networks. Total suspended solid (TSS) content of water depends on the amount of suspended particles related to turbidity of water. The present study recorded 285 mg/L for TSS, which is lower than the WHO guideline [8]. Its presence could be due to disposal of sewage and industrial effluent to the river.
|
3.2. Effect of the Contact Time
- The effects of contact time on the removal of calcium and magnesium using melon (Citrullus lanatus) husk were as shown in Figure 1. The percentage adsorption increased with increase in contact time at constant concentration until the equilibrium times were reached at 20min for calcium ion (Ca2+) and 25 min for magnesium ion (Mg2+). Before the optimum time, the removal efficiencies of both Mg (II) and Ca (II) increased rapidly due to the abundant availability of active binding sites on the sorbent surface. In the second step, the removal process became less efficient due to the complete occupation of the surface with the metal ion. The surface of adsorption can be considered as the driving force for these ions removal [18]. The equilibration time required by the melon (Citrullus lanatus) husk used in this study for the adsorption of calcium ion is less compared to the time required for magnesium ion removal. However, short equilibration time is essential for having economically treated water [26]. Shorter contact time exhibited for removal of Ca2+ has advantageous effect of reducing operation cost over Mg2+. The reason for that is likely to be due to the smaller hydrated radius of Ca ions compared to that of Mg ions which leads to Ca ions to be adsorbed faster than Mg ions [27]. This finding correlates with the study conducted by Sepehr et al. [18] that found natural pumice stone adsorbent being a better material in removing Ca ions than Mg ions from water.
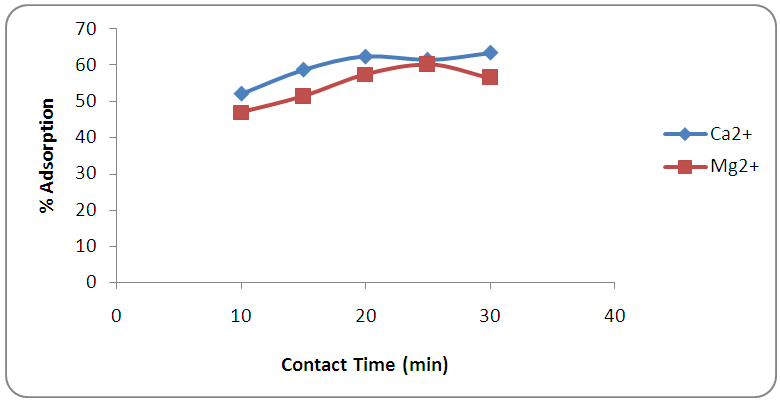 | Figure 1. Effect of contact time on the removal of calcium and magnesium ions by adsorption at 0.50 g adsorbent weight and pH 7 |
3.3. Effect of the pH
- Figures 2 and 3 show optimized pH for the removal of calcium and magnesium ions which were in the range 4-6. The pH of the solution is considered as an essential factor affecting metal adsorption process due to its impact on the degree of ionization of metal specie and the surface charge of the adsorbent [28]. At low pH values (acidic media), protons were available to protonate the active sites of the sorbent surface. While in basic media, cations will begin to precipitate as a result of metal hydroxide and the adsorption rate decreases [29]. Similar results were reported for the removal of calcium using raw sugar cane bagasse modified with tartaric acid and citric acid [30] and in the dynamic study of adsorption isotherms for the effluents [7].
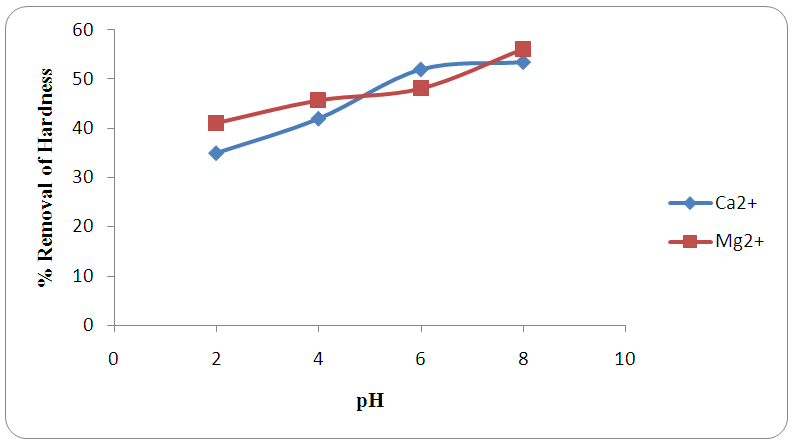 | Figure 2. Effect of pH on the removal of hardness (Ca2+ and Mg2+) by adsorption at 0.50 g adsorbent weight |
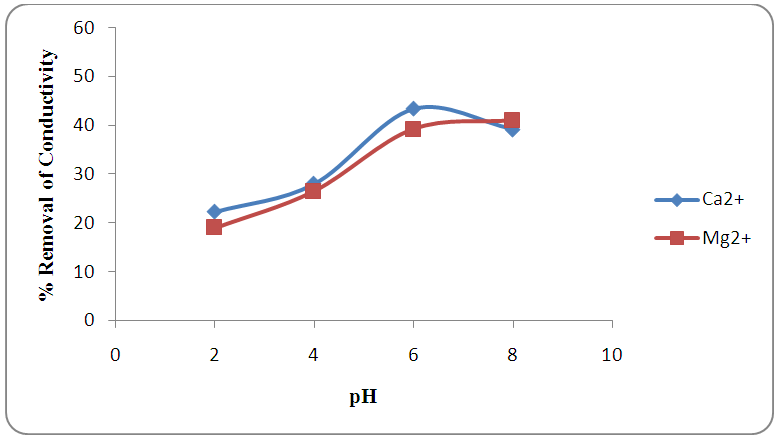 | Figure 3. Effect of pH on the reduction of conductivity by adsorption at 0.50 g adsorbents weight |
3.4. Effect of Adsorbent Mass
- The plot of the percentage adsorption of calcium and magnesium ions against the adsorbent dosage employed is shown in Figures 4 and 5. These reveal that the amounts of the metal ions adsorbed increased with an increase in the amount of activated melon (Citrullus lanatus) husk from 1.0 g to 3.0 g with 40cm3 of the water sample. It implies that the corresponding removal efficiency increases for both Ca2+ and Mg2+ with increase in adsorbent masses. These increases in the sorption of the amounts of metal ions are due to the existence of larger surface area as well as larger number of active binding sites for the ions. Similar trend was also observed by Saeed and Hamzah [14] who studied and applied commercial polyacrylic acid hydrogel beads for the removal of total hardness from water. It was obvious that at high sorbent dosage, the available metal ions are sufficient to cover the exchangeable sites on the activated melon (Citrullus lanatus) husk which results in high metal ions uptake.
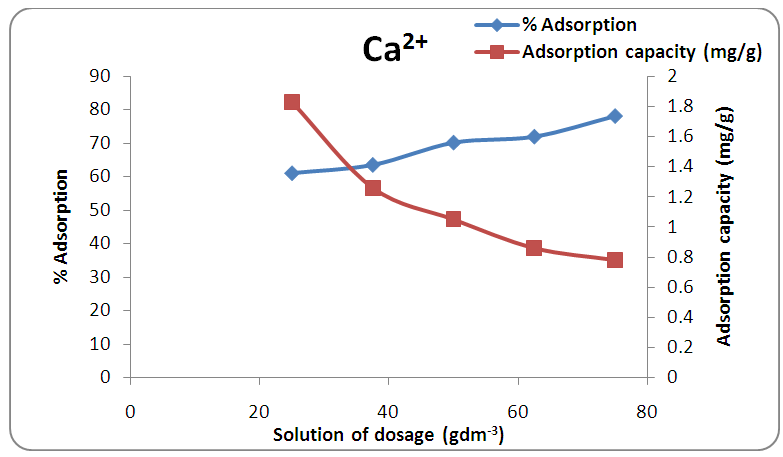 | Figure 4. Effect of adsorbent dose on the removal of calcium ion by adsorption at contact time 20 min and pH 7.0 |
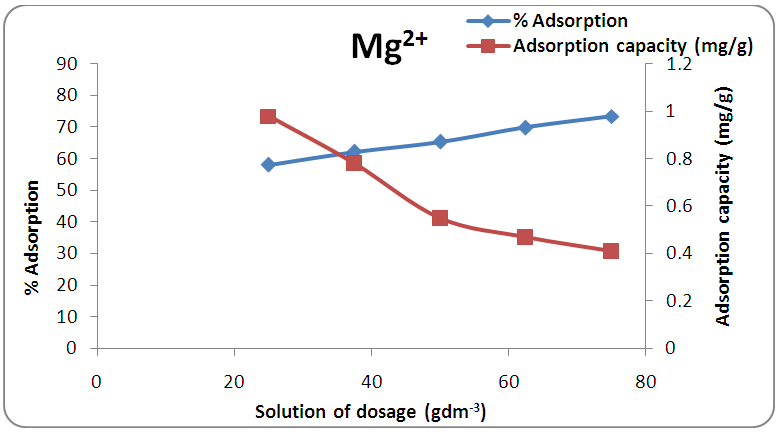 | Figure 5. Effect of adsorbent dose on the removal of magnesium ion by adsorption at contact time 25 min and pH 7.0 |
3.5. Effect of Temperature
- The removal studies were conducted at different temperatures of 30, 40, 50, 60 and 70°C. Temperature plays a major role in the adsorption of heavy metals on the surface of adsorbent and has two major effects on the adsorption process; increase in the temperature increases the rate of adsorbate diffusion across the external boundary layer and in the internal pores of the adsorbate particles as the liquid viscosity decreases with increase in temperature and the other affects the equilibrium capacity of the adsorbate. Figure 6 reveals that the removal of hardness (Ca2+ and Mg2+) increases as temperature increases, this may be due to the increase in ion mobility to the adsorbent. Temperature affects the interaction between the adsorbent and the metal ions which influences the stability of the metal–sorbent complex [31]. Higher temperatures enhance sorption due to the increased surface activities and kinetic energy of the solute. However, physical change can be expected at higher temperatures [32].
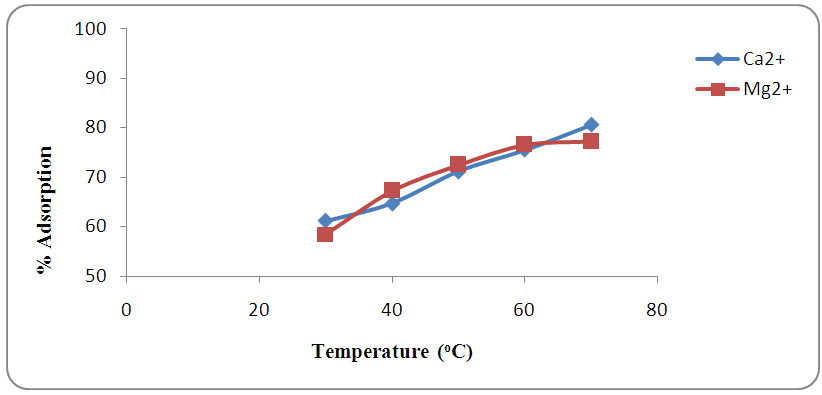 | Figure 6. Effect of temperature on the removal of hardness (Ca2+ and Mg2+) by adsorption at pH 7.0 |
3.6. Thermodynamic Parameters of Adsorption
- The thermodynamic parameters (standard free energy,
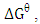 standard enthalpy change,
standard enthalpy change, 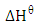 and standard free entropy,
and standard free entropy, 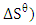 are presented in Table 2. The values of
are presented in Table 2. The values of 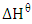 and
and 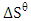 were respectively determined from the slope and intercept of the plot of
were respectively determined from the slope and intercept of the plot of 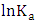 against inverse of temperature. The Gibbs free energy is calculated for adsorption of (Ca2+ and Mg2+) on activated melon (Citrullus lanatus) husk using equation (11). The
against inverse of temperature. The Gibbs free energy is calculated for adsorption of (Ca2+ and Mg2+) on activated melon (Citrullus lanatus) husk using equation (11). The 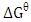 values are negative for the metal ions onto the adsorbent which indicates that adsorption is thermodynamically feasible and spontaneous. It was observed that
values are negative for the metal ions onto the adsorbent which indicates that adsorption is thermodynamically feasible and spontaneous. It was observed that 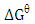 decreased with temperature and better adsorption was obtained at higher temperatures. The positive values of the standard enthalpy change
decreased with temperature and better adsorption was obtained at higher temperatures. The positive values of the standard enthalpy change 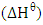 indicates that the adsorption process is endothermic. It was reported by Ozdemira et al. [31] that adsorption of
indicates that the adsorption process is endothermic. It was reported by Ozdemira et al. [31] that adsorption of 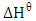 lower than 40 kJ/mol is physiosorption. It indicates that adsorption is physical by nature and involves weak forces of attraction. The positive value of
lower than 40 kJ/mol is physiosorption. It indicates that adsorption is physical by nature and involves weak forces of attraction. The positive value of 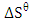 shows an increase in the randomness at the activated melon (Citrullus lanatus) husk and wastewater interface during adsorption process. Positive values of
shows an increase in the randomness at the activated melon (Citrullus lanatus) husk and wastewater interface during adsorption process. Positive values of 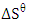 reveal the affinity of the adsorbents for Ca2+ and Mg2+ which suggests some structural changes in the adsorbate and adsorbent. Also, it was observed that correlation coefficient, R2, for magnesium ion was much lower than calcium ion. This showed that adsorption rate of Ca2+ was greater than Mg2+.
reveal the affinity of the adsorbents for Ca2+ and Mg2+ which suggests some structural changes in the adsorbate and adsorbent. Also, it was observed that correlation coefficient, R2, for magnesium ion was much lower than calcium ion. This showed that adsorption rate of Ca2+ was greater than Mg2+.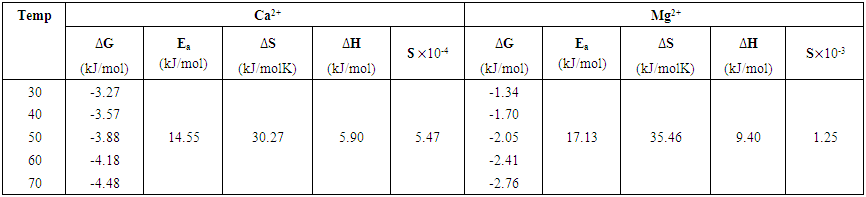 | Table 2. Thermodynamic parameters for the adsorption of Ca2+ and Mg2+ onto the activated melon (Citrullus lanatus) husk |
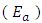 and sticking probability (S*) were calculated from the experimental data using Arrhenius equation related to surface coverage as expressed in the equation:
and sticking probability (S*) were calculated from the experimental data using Arrhenius equation related to surface coverage as expressed in the equation: 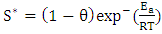 | (13) |
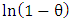 against
against 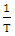 with intercepts of
with intercepts of 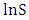 and slope of
and slope of 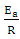 were as shown in Figure 8. The activation energy
were as shown in Figure 8. The activation energy 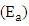 values computed from the slopes of the plots were found to be 14.55 kJ/mol and 17.13 kJ/mol for Ca2+ and Mg2+ respectively. The positive values of
values computed from the slopes of the plots were found to be 14.55 kJ/mol and 17.13 kJ/mol for Ca2+ and Mg2+ respectively. The positive values of 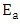 indicate that higher temperature will favour the removal of the metal ions responsible for hardness (Ca2+ and Mg2+) on the activated melon (Citrullus lanatus) husk which signifies the endothermic nature of the sorption process. The sticking probability, S*, is a function of the sorbate and sorbent system. The measurements of sticking potential of sorbate and sorbent were as shown in Table 3. The sticking probability values for the metal ions (Ca2+ and Mg2+) to the sorbent were in the range 0 < S* < 1 which were also temperature dependent. These values confirmed that the adsorption process maintained a physiosorption mechanism.
indicate that higher temperature will favour the removal of the metal ions responsible for hardness (Ca2+ and Mg2+) on the activated melon (Citrullus lanatus) husk which signifies the endothermic nature of the sorption process. The sticking probability, S*, is a function of the sorbate and sorbent system. The measurements of sticking potential of sorbate and sorbent were as shown in Table 3. The sticking probability values for the metal ions (Ca2+ and Mg2+) to the sorbent were in the range 0 < S* < 1 which were also temperature dependent. These values confirmed that the adsorption process maintained a physiosorption mechanism.
|
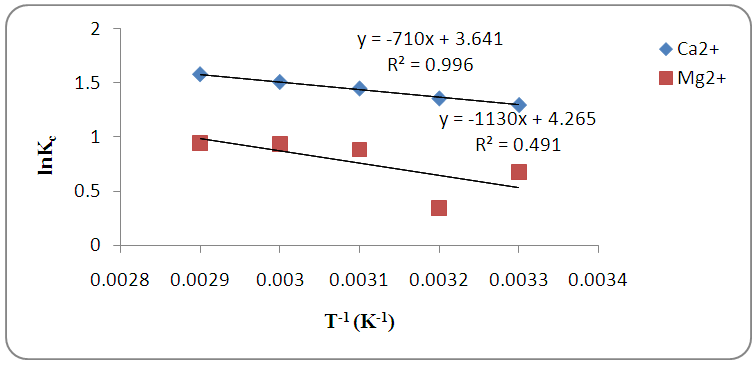 | Figure 7. Plot of lnKc against reciprocal of temperature for Ca2+ and Mg2+ adsorption on melon (Citrullus lanatus) husk |
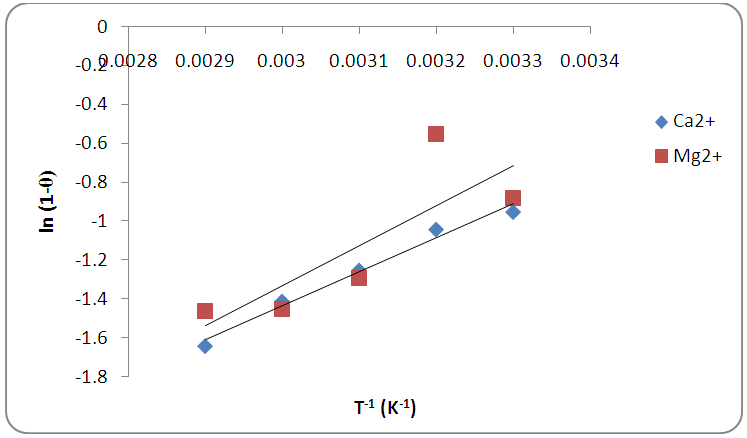 | Figure 8. The relationship between temperature and sticking probability for Ca2+ and Mg2+ adsorption on melon (Citrullus lanatus) husk |
4. Conclusions
- Physicochemical parameters of water sample collected from an urban area in Kaduna, Nigeria and removal of hardness (Ca2+ and Mg2+) were studied using activated melon (Citrullus lanatus) husks as adsorbent. Application of operational conditions such as contact time, sorbent mass, pH and temperature led to increase of hardness removal. Higher degrees of adsorption for calcium ions onto the adsorbent when compared to magnesium were observed. Thermodynamic results clearly found that adsorption of Ca2+ and Mg2+ on to activated melon husks was favoured at higher temperature and that the biosorption process is spontaneous
 and exergonic. The activation energy
and exergonic. The activation energy 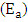 further supports higher solution temperatures, excellent sticking of the metal ions on to the adsorbent and the mechanism is physisorption. Thus, this study has showed that activated melon husk could provide another valuable material for the removal of hardness. This could be of significant interest to water treatment operations since it is environmental friendly and can also be an affordable technology for industries.
further supports higher solution temperatures, excellent sticking of the metal ions on to the adsorbent and the mechanism is physisorption. Thus, this study has showed that activated melon husk could provide another valuable material for the removal of hardness. This could be of significant interest to water treatment operations since it is environmental friendly and can also be an affordable technology for industries.  Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML