-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2015; 5(4): 69-73
doi:10.5923/j.aac.20150504.01
Optimization of Electrochemical Detection of L-Ascorbic Acid from Plant Food Supplements Using Screen Printed Transducers
M. Badea1, M. Florescu1, V. Veregut1, L. Chelmea1, O. Corcan1, L. Floroian1, P. Restani2, J. L. Marty3, M. Moga1
1Faculty of Medicine, Transilvania University of Brasov, Romania
2University of Milan, Italy
3University of Perpignan Via Domitia, France
Correspondence to: M. Badea, Faculty of Medicine, Transilvania University of Brasov, Romania.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The importance of Ascorbic acid (vitamin C) in several biological processes, and during the last years as active compounds used in several pesticides (L-Ascorbic acid is used as a fungicide on glasshouse tomato, potato, and field and glasshouse flower bulbs) is a challenge for groups of researchers to develop new, rapid and sensitive method for its detection. Methods for the detection of Ascorbic acid (AA) were developed and optimised using differential pulse voltammetry (DPV) analysis with modified carbon-printed electrodes with cobalt phthalocyanine. There was studied detection of Ascorbic acid in hydrochloric acid 2% as supporting media. Results showed that the method developed for Ascorbic acid analyses can be used to assay different samples as teas and plant food supplements containing different botanicals.
Keywords: Ascorbic acid, Differential pulse voltammetry, Botanical extracts
Cite this paper: M. Badea, M. Florescu, V. Veregut, L. Chelmea, O. Corcan, L. Floroian, P. Restani, J. L. Marty, M. Moga, Optimization of Electrochemical Detection of L-Ascorbic Acid from Plant Food Supplements Using Screen Printed Transducers, Advances in Analytical Chemistry, Vol. 5 No. 4, 2015, pp. 69-73. doi: 10.5923/j.aac.20150504.01.
Article Outline
1. Introduction
1.1. Ascorbic Acid in Biological Processes
- Widespread in green plants, especially fruit and vegetables, the highest concentrations are found in citrus fruits, underbrush, strawberries, kiwi, tomatoes, peppers, broccoli [1]. Sea-buckthorn fruit among the most enriched plant sources of vitamin C - in a range of 114 to 1550 mg per 100 grams with an average content (695 mg per 100 grams) about 15 times greater than oranges (45 mg per 100 grams) [2]. During processing and storage, vitamin C losses may occur [3-4]. Studies indicated that quercetin (antioxidant belongs to the broad flavonoids group) and vitamin C supplementation may not be improve lipid profile, although it may reduce induce muscle damage and the proportion of body fat [6,7].Vitamin C helps to synthesize the amino acid L-carnitine and the catecholamines [8] that occur in the regulation of the nervous system, promotes iron absorption by reducing, at stomach level, from Fe2+ to Fe3+ [9], lowering histamine ingested and if an allergic reaction occurs.Involved in the metabolism of tyrosine, and tryptophan, folic acid, the presence of Ascorbic acid is essential for the hydroxylation of dopamine to norepinephrine and in the reaction of tryptophan in order to obtain [10].Ascorbic acid is an important antioxidant, it has the property of being water soluble, may act as both intracellular and extracellular in order to protect the body against free radicals and pollutants [5].An amount below the lower limit of vitamin C in the body can cause anemia [11], scurvy [12], various infections [13], mental disorders [14]. Mega doses of vitamin C are used in the treatment and prevention of large number of disorders like diabetes, cataracts, glaucoma, macular degeneration, atherosclerosis, stroke, heart diseases and cancer [5].An amount above the upper limit, normally not cause toxicity due to water solubility, the excess is excreted. A normal amount of vitamin C in plasma is between 4.6 and 14.9 mg/L [15, 16].Several controversies were related to the action of vitamin C and the dosage [17]. The topic is also now very interesting for investigators and EFSA (European Food Safety Association) that launched open consultations on dietary reference values for vitamin C. The document proposed dietary reference values for Ascorbic acid for different ages (adults, infants and children), and different status of women (pregnant and lactating women) [18].Under the European law, manufacturers of food supplements are responsible for the safety of their products before they are commercialized. They are also responsible for determining that the claims on their labels are accurate and truthful.
1.2. Ascorbic Acid as Pesticide
- L-Ascorbic acid (IUPACname-((5R)-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one) is a new active substance for which received an application from Citrex Europe B.V. for approval (in accordance with Article 6(2) of Council Directive 91/414/EEC the Netherlands). The competent authority from Netherlands prepared a review concerning the initial risk assessments of the pesticide active substance L-Ascorbic acid. There have been evaluated the representative uses of L-Ascorbic acid as a fungicide on glasshouse tomato, potato, and flower bulbs (field and glasshouse) [19].The context of this peer review was that required by Commission Regulation (EU) No 188/2011. Based on those conclusions, European Food Safety Authority (EFSA) identified that:Data gaps were found for the studies concerning physical and chemical properties and analytical methods.Toxicological issues and metabolism sections were completed and no data gaps or critical concerns were identified.In the fields of residues no data gaps were identified and the risk assessment studies can be done.A low risk was observed for honey bees, non-target plants, birds, mammals, and sewage treatment organisms.On the basis of the available scientific information, a high risk to non-target arthropod was identified for their use on potatoes.The Ascorbic acid can have a double action. There were also studies reflecting the protective effect of Ascorbic acid on liver enzyme activity in rats exposed to diazinon (organophosphate pesticide). The results revealed that vitamin C have a potent beneficial (protective) effect against diazinon-induced hepatotoxicity in rats. These could be explained be due to the scavenging effect of free radicals and increased the total antioxidant status [20].Animal studies (micronucleus test in Swiss albino mice) indicated the modulatory effect of higher doses of vitamin C on the genotoxicity of the some pesticides (endosulfan, mancozeb, and phosphamidon) [21].
1.3. Detection Methods of Ascorbic Acid
- Classic method recommended for Ascorbic acid detection was presented as titrimetric method (iodometric titration in presence of amidon [22].Szalai and colab. [23] developed a simple, selective, economic, precise and stable HPLC method for the detection of ascorbate in plant tissue.Electrocatalytic study for detection of Ascorbic acid using single-walled carbon nanotube/tungsten oxide (SWCNT/WO3) modified glassy carbon electrode (GCE) prepared by mechanical attachment was conducted. The SWCNT/WO3-modified glassy carbon electrodes possess better sensitivity when compared to WO3-modified electrode and bare GCE [24].Murthy [25] proposed a modified electrode with TCNQ for Ascorbic acid oxidation in phosphate buffer solutions (pH 7) using cyclic voltammetry. The method is suitable for a quantitative determination of Ascorbic acid (linear variation of current with concentration of Ascorbic acid in the range 1–10 mM).Differential pulse voltammetry (DPV) has been used to simultaneously detect of dopamine, serotonin (5-HT), and Ascorbic acid [26]. Linear calibration curve were obtained. The linearity range for Ascorbic acid was 300.0-2,000.0 μM, with detection limits (S/N = 3) of 0.6 μM. The proposed electrode has been applied to dopamine, serotonin (5-HT), and Ascorbic acid in real samples using standard addition method. The obtained results were satisfactory.Electrochemical studies (voltammetric and amperometric methods) were done in the past by our group for detection of vitamin C, using cobalt phtalocyanine as redox mediator [27].This work proposes an alternative to titrimetric methods, usually used for detection of Ascorbic acid from food samples. The new proposed method also is innovative because use the new type of transducers prepared in the BIOMEM laboratory from University of Perpignan Via Domitia, France [28].
2. Materials and Equipment
- Analytical grade reagents were used. L-Ascorbic acid was purchased from Merck, Darmstadt, Germany. Solutions of HCl 2% were used for real sample preparation and as supporting electrolyte. Three plant food supplements (PSF) were used as real samples.Sample 1 is a PFS containing Hippophae fructus, cuticle of chicken gizzard, Chamomillae flos, Hyperici herba, Amaranthus caudatus herba, Thymi vulgaris aetheroleum, Lavandulae aetheroleum and excipients. Sample 2 is a PFS containing Hippophae fructus, Spirulina platensis, Foeniculi aetheroleum, Lavandulae aetheroleum, Menthae piperita aetheroleum and excipients. Sample 3 is a PFS containing Hippophae rhamnoides, Liquiritiae radix, Violae herba, Bardanae radix, Taraxaci herba cum radices and excipients.As electrochemical transducers there were used screen printed electrodes (SPE), obtained on laboratories of University de Perpignan Via Domitia, France [29]. Working electrodes were made from carbon (SPE) and carbon - modified with cobalt phtalocyanine (SPE- Co), as redox mediator (Fig.1). Palm Sense Potentiostate with Soft PS Trace software was used for analysis.
 | Figure 1. Design of SPE electrodes |
3. Working Procedure
- The measurements were performed at room temperature.Titrimetric measurements of Ascorbic acid were done according with procedure proposed by Cioroi [22].To measure Ascorbic acid in hydrochloric acid medium there was used DPV method with SPE-Co sensor. The working parameters were maintained constants during the experiments, according with other previous studies [22, 30, 31]. Differential pulse voltammetry (DPV) parameters were maintained as: start potential -0.6V; final potential 1V; scan rate 0.01 V/s; t pulse 0.2s; deposition time 240s.When HCl solution 2% was used as electrolyte, the final concentration of Ascorbic acid in the media was between 0.05mM and 0.25mM respectively.Calibration curves for L-Ascorbic acid in hydrochloric acid 2% were obtained (each standard sample was tested three times). Data interpolation for detection of Ascorbic acid content from real samples (plant food supplements in acidic media) was done.A quantity of 5 g plant food supplement was grind with approx. 10 cm3 solution 2% HCl and 2.5 g of quartz sand for 10 minutes. The resulting mixture was transferred into a 50 mL volumetric flask and brings to volume with HCl 2%. It is filtered through a fluted filter into a dry flask. 10 cm3 filtrate was pipetted into a 100 ml Erlenmeyer flask.
4. Results and Discussions
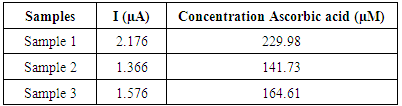
- The obtained signals using Differential Pulse Voltammetry (DPV) were proportional with the concentration of Ascorbic acid from the standard solutions. Analysis of Ascorbic acid in hydrochloric acid 2% indicated the calibration line from Fig.2.
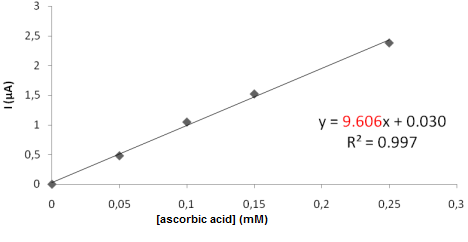 | Figure 2. The calibration curve for measurement of L-Ascorbic acid (vitamin C) in a solution HCl 2% using DPV method (sensor SPE-Co) |
|
|
5. Conclusions
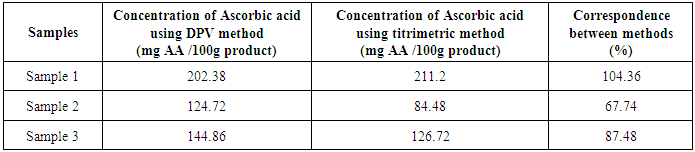
- Methods for the detection of Ascorbic acid (AA) were developed and optimised using differential pulse voltammetry (DVP) analysis with a screen printed modified carbon-printed electrodes with cobalt phthalocyanine.Results showed that the method developed for Ascorbic acid analyses can be used to assay different samples as plant food supplements as well as possible pharmaceutical samples and teas. This detection method of Ascorbic acid from real samples was possible and it was more sensitive than the recommended titration for detection of Ascorbic acid from food samples.
ACKNOWLEDGEMENTS
- The writing of this work has received partial funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 245199. It has been carried out within the PlantLIBRA project (website: www.plantlibra.eu). This report does not necessarily reflect the Commission views or its future policy on these areas.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML