-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2015; 5(2): 42-49
doi:10.5923/j.aac.20150502.03
Chemical Composition and in vitro Antioxidant Activities of Ocimum americanum
Geoffrey Muriira Karau 1, 2, Eliud Nyagah Mwaniki Njagi 2, Alex King’ori Machocho 3, Laura Nyawira Wangai 4, Mungiria Juster Nthinga 5
1Eagleways Technologies Ltd, Nairobi, Kenya
2Department of Biochemistry and Biotechnology, Kenyatta University, Nairobi, Kenya
3Department of Chemistry, Kenyatta University, Nairobi, Kenya
4Kirinyaga University College, School of Health Sciences, Kerugoya
5Department of Nursing, Chuka University
Correspondence to: Geoffrey Muriira Karau , Eagleways Technologies Ltd, Nairobi, Kenya.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This study evaluated chemical composition and in vitro antioxidant activity of Ocimum americanum. Standard methods were used in determination of phytochemicals and high performance liquid chromatography (HPLC) with UV visible detector was used in analyses of carotenoids and vitamins. Chemical compounds in ethyl acetate extracts were determined using GC-MS with National Institute of Standards and Technology (NIST), 2011 chemical compounds database. Antioxidant activity was determined by 1, 1-diphenyl-2-picrylhydrazyl (DPPH), phosphomolybdate and reducing power assays. O. americanum contains diverse chemical compounds and the antioxidant activity was high. The ethyl acetate extracts contained 69 chemical compounds ranging from phytosterols, terpenes and fatty acid derivatives, among many others. These chemical compounds and antioxidant activity could be responsible for the efficacy in management of many diseases among the Kenyan societies.
Keywords: Antioxidant activity, Phytochemicals, Ocimum americanum, DPPH assay, Phosphomolybdate assay
Cite this paper: Geoffrey Muriira Karau , Eliud Nyagah Mwaniki Njagi , Alex King’ori Machocho , Laura Nyawira Wangai , Mungiria Juster Nthinga , Chemical Composition and in vitro Antioxidant Activities of Ocimum americanum, Advances in Analytical Chemistry, Vol. 5 No. 2, 2015, pp. 42-49. doi: 10.5923/j.aac.20150502.03.
Article Outline
1. Introduction
- The use of medicinal plants is embedded in the human cultural fabric ever since the times of early man. Medicinal plants are a source of a wide and diverse variety of phytochemicals that are of great medical importance. Knowledge of the chemical constituents of plants is desirable, not only for the discovery of therapeutic agents, but also because such information is invaluable in availing new chemical resources [1]. The World Health Organization (WHO) emphasizes on the use of traditional medicines and has reported that about 80% of population from developing countries relies on medicinal plants for their primary health care [2, 3]. Popular and scientific observations on the safety and efficacy of medicinal plants significantly contribute to discovery of their therapeutic potential and search of active chemical constituents. According to WHO, despite existence and continued use of medicinal plants overmany centuries, they have not been officially recognized in most countries. The main reason for this would be lack of reliable research data and acceptable methodology on the medicinal plants for proper evaluation of their counterparts and claims by medicinal plants practitioners and herbalists [1]. Consequently, there is an urgent need for research, training and education on medicinal herbs especially on the composition, safety and efficacy to tap the multiple potentials they possess, and provide a criteria for their evaluations and standardization. Ocimum americanum L. (syn. O. canum Sims) belongs to the family Lamiaceae, a plant species widely distributed in the tropics and sub-tropics of the Old and New World. Contrary to its name it is not native to America, but only to Africa and Asia [4]. It is referred to as “Makuruthi” by Ameru and “Mutaa” by Mbeere people of eastern part of Kenya. The essential oils found in this species have strong fungicidal activity against certain plant pathogens [5]. In Africa, leaves of O. americanum are used as an insecticide for the protection against post-harvest insect damage especially the ones caused by bruchid beetles [6]. The active ingredients are also likely to be associated with the essential oils. However, some medicinal properties may be associated with the external flavonoids, as some specimens produce very high levels of these compounds, especially nevadensin, which has antioxidant activity [7]. This study evaluated the qualitative and quantitative chemical composition, and in vitro antioxidant activities of O. americanum. The current study supports the use of O. americanum in management and treatment of various diseases based on its chemical compositions and helps in accurate formulations of products that are efficacious and stable for consumpution.
2. Materials and Methods
2.1. Collection of Plant Materials
- Leaves and stems of Ocimum americanum were collected from their geo-ecological habitats in Mbeu village, Tigania, Meru County of Kenya based on their forkloric use as was obtained from traditional medical practitioners. The plants were authenticated by a Taxonomist at the Department of Plant and Microbial Sciences, Kenyatta University, Kenya and a voucher specimen deposited at the Kenyatta University Herbarium for future reference.
2.2. Preparation and Extraction of Plant Materials
- The plant materials were processed according to methods in our laboratory and as described elsewhere [8]. Briefly, 100 g of plant materials was extracted in 1 L of distilled water. Similarly, 100 g was extracted in 1 L of ethy acetate and the extracts freeze dried, weighed, and stored in clean air-tight polythene bags for further analyses.
2.3. Qualitative and Quantitative Determination of Chemical Compounds
- Preliminary phytochemicals screening and quantitative determination was carried out according to the methods described elsewhere [8]. The following phytochemicals were screened and quantified; flavonoids, polyphenols, alkaloids and tannins among others. Screening of chemical compounds in ethyl acetate extract was carried out using gas chromatography mass spectrometry (GC-MS) according to methods published in our laboratory [9]. The chemical compounds were identified based on their mass spectra by deconvolution of the chromatographs and matching with the compounds in the NIST 2011 database [9]. The following vitamins and carotenoids were quantified using reverse phase high performance liquid chromatography (HPLC) fitted with UV/Vis detector; vitamin B1, B3, retinol, tocopherol, ascorbic acid, β-carotene, lycopene, and β-cryptoxanthin; according to method described elsewhere in our laboratory [8].
2.4. Antioxidant Activity
- Antioxidant activity was determined according to the following three methods; 1, 1-dipheny-2-picrylhydrazyl (DPPH), Phosphomolybdate and reducing power assay, as described elsewhere [8].
3. Results
3.1. Qualitative and Quantitative Phytochemicals
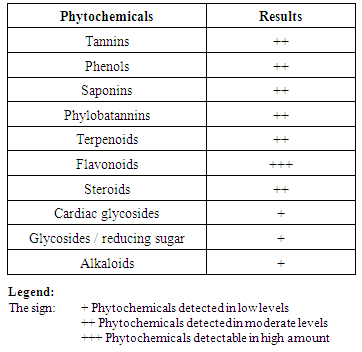
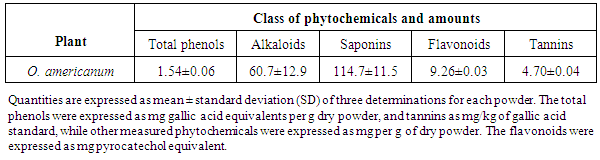
- The ethyl acetate and aqueous extracts yielded a dark brown and a light brown paste of concentrations of 215.7 and 168.0 mg/g dry weight, respectively. The following phytochemicals were found in the plant materials; tannins, phenols, and saponins among others as shown in Table 1. For the quantitative composition, it was found that saponins were the highest with 114.7 ± 11.5 mg per gram dry powder, while the lowest was tannins at 4.70 ± 0.04 mg per g of dry materials. The total phenols yield was 1.54 ±0.06 mg gallic acid equivalent per gram of dry powdered material, as shown in Table 2.
|
|
3.2. Nutritional Composition
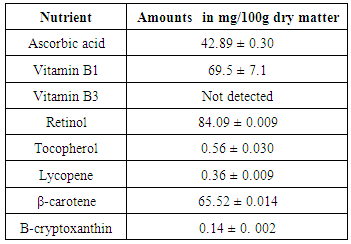
- The amounts of nutrients were determined using reverse phase HPLC-UV vis detector. The nutrients determined were both conjugated and non-conjugated carotenoids and vitamins A, E, B1, B3 and C. It was found that retinol was the highest in concentrations at 84.09 ± 0.009 mg/100 g, followed by vitamin B1 at 69.50 ± 7.1 mg/ 100 g of dry powder. Vitamin B3 was not detected. Other nutrients quantified include lycopene, β-carotene, β-cryptoxanthin, tocopherol as shown in table 3.
|
3.3. Compounds from Ethyl Acetate Extracts
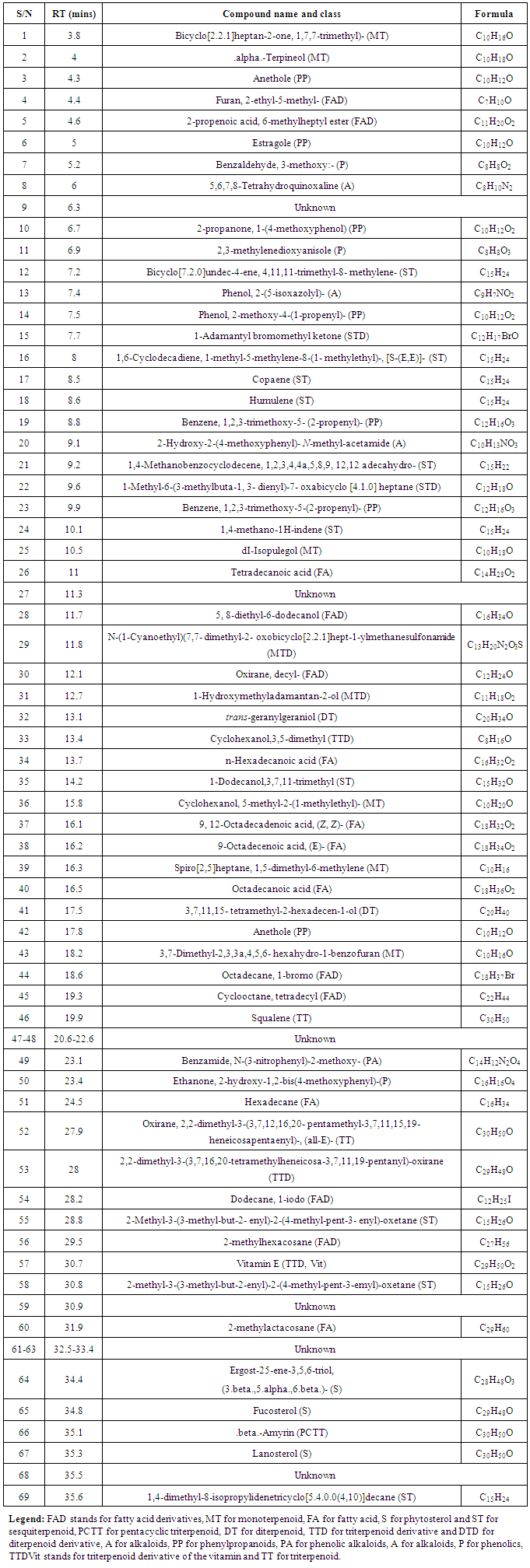
- As depicted in Table 4 and Figure 3.4, 69 diverse chemical compounds were detected from ethyl acetate of O. americanum. These compounds range from phytosterols, fatty acids and fatty acids derivatives, terpenoids, alkaloids and phenolics among others, whose fragments were repeated severally in the chromatographs. We could not establish the identity of nine (9) compounds since they were not available in the NIST11 chemical compounds database.The most abundant compound was benzaldehyde, 3-methoxy, a phenolic followed by 2,2-dimethyl-3-(3,7,16,20-tetramethylheneicosa-3,7,11,19-pentanyl)-oxirane, a triterpenoid derivative and then 3,7,11,15- tetramethyl-2-hexadecen-1-ol, a diterpenoid as demonstrated by the heights of their chromatograph peaks in the figure 3.4. Of the nine (9) unknown compounds, 9, 61 and 68 were the most abundant. Their spectra images from deconvolution of their chromatograms are shown in Figure 3.1, 3.2 and 3.3. Compound 9 eluted at 6.3 minutes and had the charge to mass ratio (m/z) of 164.12, and in the entire scan this was designated as compound 12 because many fragments of the chemical compounds were repeated in the scan. Compound 61 eluted at 32.5 minutes and its m/z was 314.16, and its spectrum is shown in figure 3.2. The compound 61 has the highest peak height, an indication that it is the most abundant of all the unknown chemical compounds in O. americanum. Compound 68 eluted at 35.5 minutes and its m/z was 444.35 and was the second most abundant unknown compound. The unidentified compound 68 by NIST2011 database could be cyclohex-2-ene-1, 4-diyl bis (4-nitrophenyl) dicarbonate with molecular mass of 444.35, and occurs in trans and cis forms and its spectra is shown in figure 3.3 [10]. This compound is classified among the naturally occurring fungicides for many crops and has insecticidal properties [11].
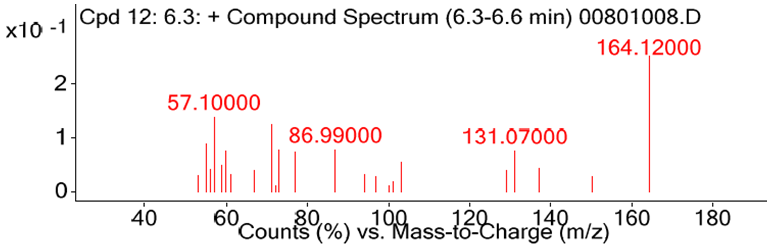 | Figure 3.1. The spectrum of the unknown compound 9 |
 | Figure 3.2. The spectrum of compound 61, with m/z ratio of 314.16 |
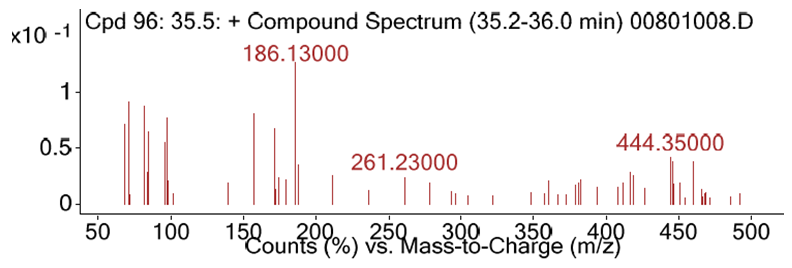 | Figure 3.3. The spectrum of compound 68, with m/z ratio of 444.16 |
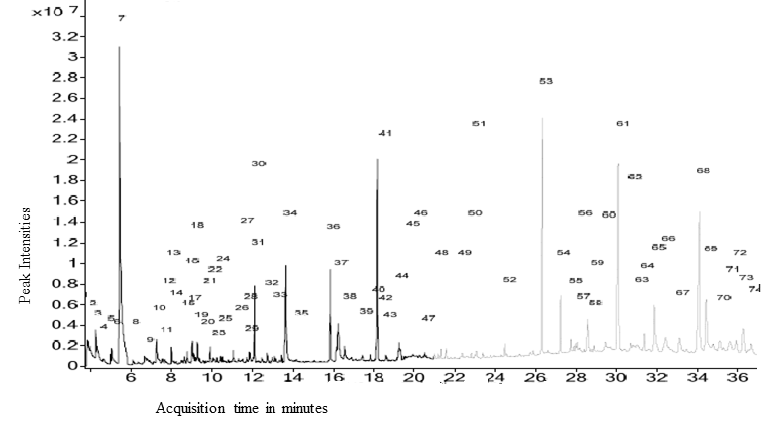 | Figure 3.4. GC-MS chromatogram of chemical compounds from ethyl acetate extracts of O. americanum |
|
3.4. Antioxidant Activity
3.4.1. Free Radical Scavenging Activity by DPPH Assay
- The effect of antioxidants on DPPH is thought to be due to their hydrogen donating ability. The antioxidant activity of O. americanum was 50.47 ± 0.03% compared to 97.76 ± 0.03% of ascorbic acid reference. The observed activities indicate that the plant powders have a strong proton-donating ability and could serve as free radical scavengers, acting perhaps as primary antioxidants.
3.4.2. Total Antioxidant Activity by Phosphomolybdate Assay
- The phosphomolybdate assay is based on the reduction of Mo (VI) to Mo (V) by the plants extracts and the ascorbic acid fractions and subsequent formation of a green phosphate Mo (V) complex at the acid pH. O. americanum total antioxidant activity was 60.08 ± 0.11 ascorbic acid equivalent (AAE) in µg/mg of dry powder.
3.4.3. Reducing Power
- As depicted in figure 3.5, a sequential increase in reducing power demonstrated by increase in absorbances with increase in the concentration of gallic acid and plants extract was observed. This is as a result of continued formation of a blue Fe2+ complex. O. americanum demonstrated a rapid and significantly high reducing potential, which reached the maximum level at the concentration of 400 µg/mL that was slightly lower than that of the gallic acid standard even in low concentrations.
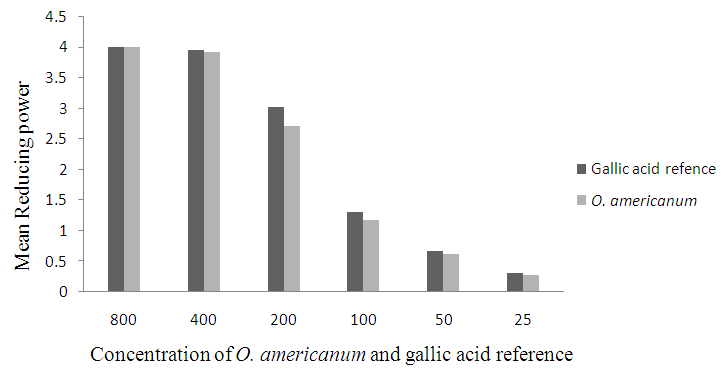 | Figure 3.5. Plots of absorbance against concentration of the gallic acid reference and O. americanum powder ranging from 25-800µg/mL |
4. Discussion and Conclusions
- O. americanum contained phytochemicals: phenols, tannins, saponins, flavonoids, alkaloids, terpenoids, phylobatannins, cardiac glycosides, sterols, fatty acids and reducing sugars; phytonutrients: vitamins C, E, B1, B3, retinol, β-carotene, lycopene and cryptoxanthin among other diverse and complex chemical compounds. Natural products have complex molecular structures, with cyclic semi-rigid scaffolds, several chiral centers, more than five H-bond donors, more than ten H-bond acceptors, more than five rotatable C-C bonds, a large polar surface area, and a molecular weight above 500 [12]. These properties are critical in their suitability as therapeutic agents because the aid in absorption and transport to the target sites. Strong antioxidant activities in medicinal plants are due to the presence of phenolic and polyphenolic compounds [13]. Free radicals scavenging effects of tea polyphenolic compounds supports their use in the management of diseases such as coronary heart disease, hypertension, diabetes and cancer [13, 14]. The expression of antioxidant enzymes and other detoxifying enzymes can be regulated by oxidative stress and by low concentrations of a broad variety of chemical agents which includes antioxidants [15]. Antioxidants such as flavonoids, vitamin C, vitamin E, lycopene, β-carotene, and cryptoxanthin present in O. americanum are important in neutralizing the reactive oxygen species (hydroxy and superoxide radicals, hydrogen peroxide, lipid peroxides) and help to maintain optimal cellular and systemic health [16]. These antioxidants interact and synergistically stabilize, or deactivate, free radicals before they attack cells. Glutathione and vitamin C work interactively to quench free radicals and have a sparing effect on each other. Vitamin E neutralizes hydrogen peroxide, and lipid peroxides and is the most effective chain-breaking antioxidant within the cell membrane where it protects membrane fatty acids from lipid peroxidation. Vitamin C regenerates vitamin E [16]. Beta carotene and other carotenoids provide antioxidant protection to lipid-rich tissues by working synergistically with vitamin E. A diet that is excessively low in fat may negatively affect beta carotene and vitamin E absorption, as well as other fat-soluble nutrients. Flavonoids neutralize hydroxy and superoxide radicals, hydrogen peroxide and lipid peroxides thereby acting as protectors against a wide range of environmental stress and in humans and animals, they function as biological response modifiers. In addition to an antioxidant effect, flavonoid compounds may exert protection against heart disease through the inhibition of cyclooxygenase and lipoxygenase activities in platelets and macrophages. Antioxidants are responsible for most of the biological activities of phytochemicals including antidiabetic, anti-inflammatory, anti-allergenic, antiviral, anti-aging and anti-carcinogenic activities [17]. The pentacyclic triterpenoids (PCTT) identified includes; beta-amyrin, lup-20 (29)-en-3-one and fern-7-en-3.beta-ol, while phytosterols includes; stigmasterol, and lanosterol. The phytosterols are mainly triterpenes which are integral structural components of plants cell membranes, and free sterols involved in stabilizing phospholipid bilayers [18]. Most of the phytosterols contain 28 or 29 carbon atoms and one or two carbon-carbon double bounds, typically one in the sterol nucleus and sometimes a second one is the alkyl side chain [18]. n-hexadecanoic acid is an essential oil demonstrated to possess antifungal, anthelminthic and antimicrobial activities [19]. Stigmasterol has been shown to reduce tumor yields and counteract the proliferative changes associated with carcinogenesis [19]. The unknown chemical compounds irrespective of their amounts could be important therapeutic agents and a source of the biological activities claimed for among traditional healers.In conclusion, the many diverse chemical compounds identified and some quantified, the in vitro antioxidant activity of O. americanum, makes it a holistic plant for use in traditional medicine and for aesthetic value among the local communities in Kenya. There is a need to carry out bioassay guided fractionation of the extracts to determine the ones that are biologically active.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the Kenya Bureau of Standards management for the support including the use of their facilities for analytical work. This study was financially supported by the National Council of Science and Technology (NCST), Kenya, through a grant number NCST/5/003/2nd CALL PhD/102.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML