-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2015; 5(1): 17-24
doi:10.5923/j.aac.20150501.03
Determination of Atorvastatin Pharmacokinetic Parameters by LC/MS-MS with Traditional Liquorice Beverage
Teeba Al. Shaikhli 1, Wael Abu Dayyih 1, Eyad Mallah 1, Mohammed Hamad 2, Nidal Qinna 1, Tawfiq Arafat 1, 3
1Department of Pharmaceutical Medical Chemistry and Pharmacognosy Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan
2Department of Basic Science, Collage of Science and Health Professions, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
3Jordan Center for Pharmaceutical Research, Amman, Jordan
Correspondence to: Wael Abu Dayyih , Department of Pharmaceutical Medical Chemistry and Pharmacognosy Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
s The objective of this randomized controlled study was firstly, developing and validating a simple and simultaneous LC/MS method to quantify Atorvastatin in rat serum. Secondly, investigating the effects of Liquorice fresh beverage on the pharmacokinetics of Atorvastatin in serum rats. Atorvastatin was determined in rat's plasma using high resolution, sensitivity and validated Liquid Chromatography–Mass Spectrometry (LC-MS); The mobile phase was (75% methanol, 25% of 0.2% Formic acid in water), ACE 5 C8 Column (50 X 2.1 mm, 5µ), 1.0 ml/min flow rate, 5 microliters Autosampler injection volume, and Glimepiride as an internal standard. The analytical method was linear with acceptable recovery, precision and accuracy. A significant (P<0.05) effect of single dose of Liquorice after 2 hours from administration of Atorvastatin, and significant effect of repeated doses of Liquorice after 6 hours from administration of Atorvastatin. There was no significant effect (P>0.05) of single dose Liquorice juice on Atorvastatin Cmax (29.847 ng/ml), and repeated doses of Liquorice the Cmax was (40.113 ng/ml) which is also considered as a non- significant effect (P>0.05) We conclude that the effects of Liquorice beverage on Atorvastatin varies between single and repeated doses and this can be a result and could affect Atorvastatin pharmacokinetics.
Keywords: Liquorice, Atorvastatin, LC/MS. Rat Plasma, Validation, Pharmacokinetic
Cite this paper: Teeba Al. Shaikhli , Wael Abu Dayyih , Eyad Mallah , Mohammed Hamad , Nidal Qinna , Tawfiq Arafat , Determination of Atorvastatin Pharmacokinetic Parameters by LC/MS-MS with Traditional Liquorice Beverage, Advances in Analytical Chemistry, Vol. 5 No. 1, 2015, pp. 17-24. doi: 10.5923/j.aac.20150501.03.
Article Outline
1. Introduction
- Atorvastatin is a synthetic second generation3-hydroxy-3- methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor which lowers plasma cholesterol levels by inhibiting theHMG-CoA reductase that catalyzes the conversion of HMG-CoA to mevalonate, an early and rate-limiting step in cholesterol biosynthesis in the liver [1, 2]. Atorvastatin is metabolized in the body by cytochrome P450 3A4 enzyme system (abbreviated CYP3A4).Atorvastatin is widely used in many countries in the treatment of severe familial or non-familial hypercholesterolemia [3, 4]. Hypercholesterolemia is known as the imbalance of plasma cholesterol levels and it is considered as a high-risk factor for inducing atherosclerosis and cardiovascular diseases (CVD) [5, 6]. CVDs are known as a major cause of death for both genders all over the world [6, 7].Many studies indicated the inverse effect of interaction of the Atorvastatin with other drugs, food, beverages or dietary supplements on its effect and activity [8-10]. Recently, many research groups illustrated the alterations in metabolism of many drugs by known popular juices like grapefruit, orange, and pomegranate juices [10-15]. Generally, drug – food interactions and in particularly, drug – beverages interactions are the most causes of alterations of pharmacokinetics or pharmacodynamics of drugs [9], either by inducing or inhibiting the enzymes found in gut or liver that may affect drugs; plasma concentrations, metabolism, absorption and availabilities [9]. Aqueous Liquorice root extract is one of the most widely used juices worldwide especially in western countries as multi-component and multi-target agents, and may exert a holistic treatment to multi-factorial diseases [15-17]. Since some fruit juice affect CYP450, administration of Atorvastatin with fruit juices particularly Liquorice can alter its pharmacokinetics [18-21].In this study, our aim was to develop and validate a simple and simultaneous analytical method for determination of Atorvastatin in rats plasma by using liquid chromatography – mass spectrometry LC/MS, and also study the effect of Liquorice fresh beverage on the pharmacokinetic profile of Atorvastatin in rats plasma and enhance the use and efficacy of rational drug therapy provided with an example of beverage -drug interactions that may have a deleterious effect on health.
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
- Deionized Water, Nanopure (Fisher Sientific).Rats Serum, (Harvested from Rats of University of Petra Animal House). Methanol advanced gradient grade (Fisher Scientific). Formic acid (GPR, Rectapur). Acetonitrile (ACN) (Fisher Scientific). Atorvastatin raw material (was a kind of gift from Dar al Dawa Pharma). Glimepiride raw material. Crushed liquorices root (purchased from local supermarket).
2.1.2. Instrumentation
- An API Mass Spectrometer was used and composed of the following: On-line vacuum Degasser (Agilent 1200). Solvent delivery systems pump (Agilent 1200). Autosampler (Agilent 1200). Thermostat column compartment (Agilent 1200). API 4000 Mass Spectrometer. ACE 5 C8 (50 x 2.1 mm, 5µm). Computer systems, Windows XP, SP3 Data Management Software 1.5.2. Other instruments used were: Bath Sonicator Crest model-175T (Ultra Sonics CORP), Sartorius balance BP 2215 and Centrifuge (Eppendorf 5417C).
2.1.3. Animals
- Spargue Dawley (S.D) adult male rats supplied by the animal house of University of Petra with a weigh range between 200- 280 g were used in the experiments. Rats were placed in air-conditioned environment (20-25℃) and exposed to a photoperiod cycle (12 hours light / 12 hours dark) daily. All animal experiments were performed in compliance with Federation of European Laboratory Animal Science Association (FELASA) guidelines and the study protocol was approved by the Research Committee (September 2013) at the Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan.
2.2. Methods
2.2.1. Experimental Procedures
- To find the effect of fresh Liquorice juice on Atorvastatin, the experimental procedure was done after preparation of the drug solution, and Liquorice beverage, the rats received a certain amount orally by a syringe (Oral gavages) (according to their weights). The rats were divided into 14 groups, each group contains six rats. First four groups were administrated with Atorvastatin only as control sample. Of a second four groups; twelve hour fasted- rats, two groups were administrated with single dose of1 ml distilled water as a control and the other two groups with 1ml of Liquorice juice. Then all groups take 1 ml of Atorvastatin. Two groups of (a twelve hour fasted six rats) administered with Liquorice (1ml each time) twice daily before the day of experiment and a single dose at the day of the experiment. The same procedure has been done with the other two groups but with distilled water (1ml each time). Four groups of rats (each group has six rats) two groups were administered with Liquorice (1ml each time) twice daily before the day of the experiment, then a single dose (1ml) administration at the day of the experiment. The same procedure will done with other two groups also but with distilled water (1 ml each time). After giving the dose of drug to the rat we took a few drops of blood in eppendorff tubes after 30 min and 1, 2, 3, 4.6 hours and in the last experiment for 12 hours of administration. The blood samples in the eppendorff were centrifuged for 5 -10 minutes to obtain the serum (100-125 µl) which was placed in labeled eppendorff tubes and stored in freezer at (-30℃) until the time of analysis.
2.2.2. Preparation of Atorvastatin Solution to be Given Orally for Rats
- A sample with a weight of 0.29 g Atorvastatin raw material was dissolved in 10 ml methanol, and then 1ml was taken and completes the volume with distilled water to100ml to get 0.29 mg/ml.
2.2.3. Plant material and Extract Preparation
- The preparation of fresh Liquorice beverage was carried out by maceration. The strainer was filled with 20 grams of plant material in 1liter of distilled cold water. The macerate was left for 2 hours then filtered to get clear beverage and freshly used.
2.2.4. Preparation of Stock Solutions for the Analytical Method Validation
2.2.4.1. Preparation of Stock Solution of Atorvastatin
- Atorvastatin working standard with a weight equivalent to 10.00 mg of dissolved it in 200 ml of methanol to get concentration 50 µg/ml stock solution of Atorvastatin.
2.2.4.2. Preparation of Stock Solution of Glimepiride as Internal Standard
- Glimepiride working standard of a weight equivalent to 10.0 mg of dissolved it in 100 ml of ACN to get concentration of 100.0 µg/ml stock solution of Glimepiride.
2.2.4.3. Preparation of Working Solution of Glimepiride I.S
- Ten µl was taken from Glimepiride stock solution (100.0 µg/ml) and was diluted to 100 ml of ACN which was considered to be I.S working solution that contains 10.0 ng/ml of Glimepiride.
2.2.4.4. Mobile Phase
- The mobile phase is consisted from aqueous solvent of formic acid as buffer (25% of the mobile phase is 0.2% formic acid in water of a mixture) and organic solvent which was methanol (75% of the mobile phase). All the chromatographic and mass detector conditions are mentioned in (Table 1).
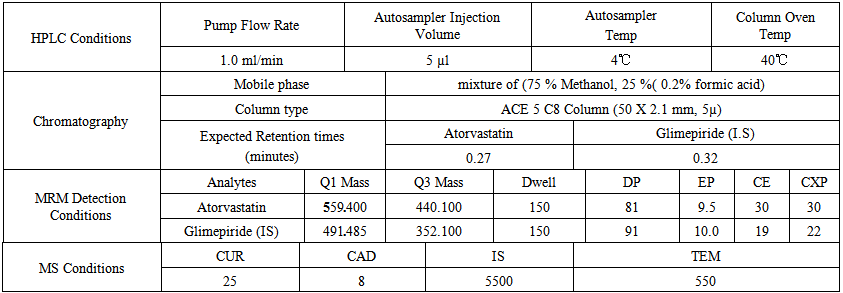 | Table 1. Summery table of chromatographic conditions and mass spectrometric conditions |
2.2.4.5. Preparation of Atorvastatin Standard Calibration Curve Serial Dilution and Spiked Serum
- Samples of standard curve in serum were prepared by spiking 20 μl from serial solution into 1 ml of serum, using seven concentrations, not including zero to obtain Atorvastatin standard calibration curve concentrations of 0.500, 1.00, 2.00, 5.00, 8.00, 12.00, 18.00 (ng/ml) in serum. Each concentration of the serum sample was divided to 50.0μl in 150.0 ml eppendorff tube and kept (-30℃). Standard samples were given daily together with the quality samples as shown in (Table 2).
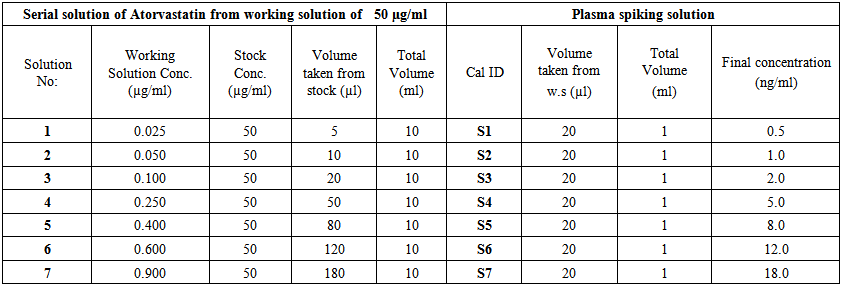 | Table 2. Preparation of Atorvastatin serial spiking samples in plasma |
2.2.4.6. Preparation of Atorvastatin Quality Control Serial Dilution and Spiked Serum
- Quality control (QC) samples in serum were prepared by spiking 20μl from serial into 1ml of serum in order to obtain quality control Atorvastatin concentrations of 1.5, 7.5, 14.0 μg/ml. Each concentrations of the QC serum sample was divided to 50.0μl in 150.0 ml eppendorff tube and kept (-30℃). QC samples were given daily together with the quality samples as shown in (Table 3).
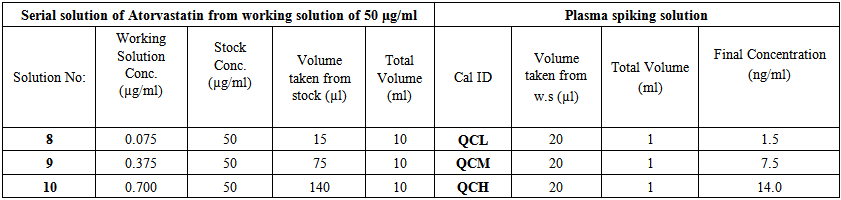 | Table 3. Preparation of Atorvastatin quality control samples in plasma |
2.2.4.7. Method of Extraction
- The procedures described were applied for subject samples, calibrator and quality control samples. In order to perform the sample extraction, the following experimental procedure was followed: Place the appropriate number of disposable Eppendorff tubes in a rack. The tubes should be properly labeled. Pipette 50.0 µl aliquots of each test sample (blank, zero, standards, QCL, QCM, QCH or Rat samples) into the appropriate tubes. Add 150.0 µl of Internal Standard (10 ng/ml glimepiride) Vortex each sample vigorously for 1.0 min. Centrifuge at 14000 rpm for 15 minutes.
2.2.4.8. Analytical Method Validation
- Method validation of performed in three separate days. In each day, seven standard calibration levels were prepared (not including zero). The prepared serum samples of the method validation represented blank, zero, standard calibration curve, six replicates of QC samples (LLOQ, Q.C Low, Q.C Mid and Q.C High). The validation parameters (linearity, intra and inter-day accuracy and precision, sensitivity and recovery) should be within the expected limits by the Food and Drug Administration (FDA) Guidance for Industry and United state pharmacopeia (USP) [21-23].
3. Results
3.1. Validation
- Validation of this analytical HPLC/MS method was performed to be evaluated in terms of recovery, linearity of response, precision, accuracy, and sensitivity for quantification of Atorvastatin. The precision of the predicted measurements for Atorvastatin was good (mean Coefficient of variation % <10%). Intra-day accuracy data showed an accuracy range of 89.411%-108.867%, 101.006%-109.200%, 96.144%-109.067% for the first, second, third day of the validation. The accuracy for Atorvastatin over all the three days of validation and all the four tested target concentration was within the accepted criteria (101.79%). The standard curve for Atorvastatin matched the requirements, linear relation (R2) ranged between; (0.996 to 1). The plot of linearity of calibration curve levels for Atorvastatin quantification against their analytical response and regression linear equation which represent all the three days of validation was done by plotting of the calculated mean of the measured concentrations versus the mean of the AUC ratio for each standard point showed in (Figure 1).
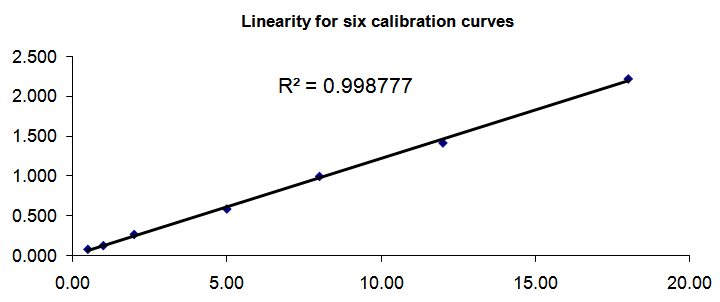 | Figure 1. The plot of linearity of calibration curve levels for Atorvastatin quantification against their analytical response and regression linear equation Stability |
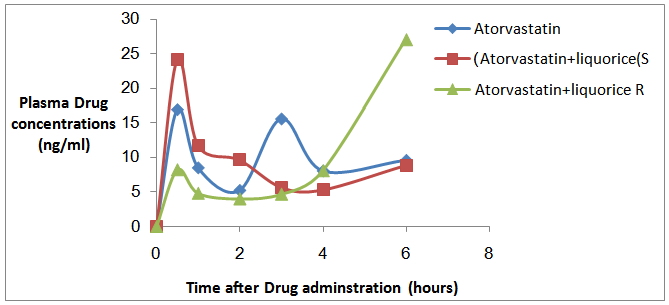
3.2. Effect of Single and Multiple dose of Liquorice Beverage on Atorvastatin
- The difference between Cmax of Atorvastatin alone and Atorvastatin with single dose administration of Liquorice is insignificant (P>0.05) as showed in (Table 4). Also for Atorvastatin with repeated doses of Liquorice, Cmax difference between Atorvastatin alone (control group) and Atorvastatin with repeated doses of Liquorice were insignificant as shown in the (Table 4). For the AUC, the difference between Atorvastatin alone and Atorvastatin with single dose of Liquorice is insignificant (P>0.05). Also, Cmax's difference between Atorvastatin alone and Atorvastatin with repeated doses of Liquorice were insignificant (P>0.05) as shown in the (Table 4).
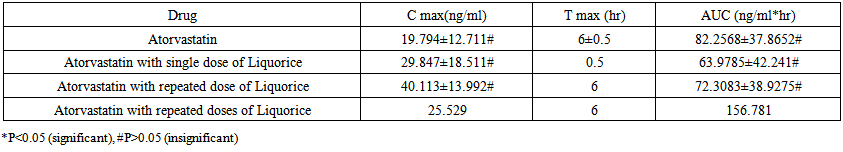 | Table 4. Pharmacokinetics data of Atorvastatin |
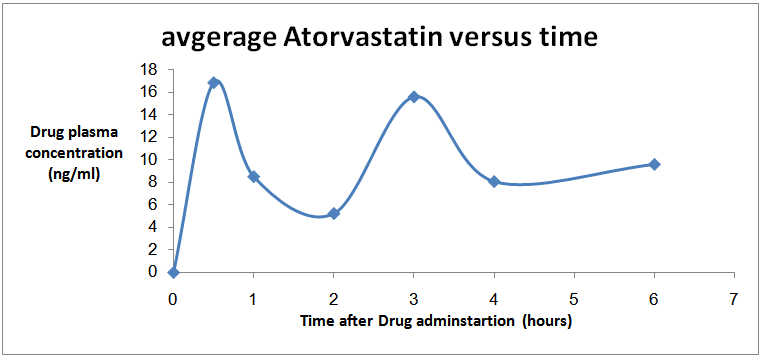 | Figure 2. Rat plasma profile showing the changes in mean serum Atorvastatin concentrations with time after drug administration, each data point represents the mean ±SEM (n=8) |
4. Discussion
- Several plant-derived beverages have been shown to modulate enzymes and transporters in the intestine, that can altered pharmacokinetic (PK) and potentially negative pharmacodynamics (PD) outcomes. Commonly consumed fruit beverages mainly; orange, grape fruit, Liquorice, and pomegranate, and teas, and alcohols drinks contain phytochemicals that could inhibit intestinal cytochrome P450 and phase II conjugation enzymes, as well as uptake and efflux transport proteins [18].The current study aimed to develop a new validated method for quantification of Atorvastatin in rats plasma and to study the effect of single and repeated doses of Liquorice beverage on Atorvastatin serum concentration in pre fed rats in comparison with rats pre fed with distilled water, to provide safe and efficient treatment on one of the most widely used chronic drug (Atorvastatin).The results showed insignificant elevation effect of single dose pre-treatment with Liquorice juice on the pharmacokinetic profile of Atorvastatin. In contrast, the repeated dose administration of Liquorice juice showed insignificant declining in the pharmacokinetic parameters. The Cmax of administration of Atorvastatin only and in combination with single dose of Liquorice and in combination with repeated doses of Liquorice were; (19.794 ng/ml), (29.874 ng/ml), and (40.113 ng/ml) respectively. There were no significant differences in Cmax's and AUC's between single administration and combination with single dose of Liquorice and between single administration and combination with repeated doses of Liquorice as showed in table 4.Atorvastatin metabolism is determined by the activity of CYP3A4 isoenzymes. Concomitant administration of Atorvastatin with fruit juices can alter Atorvastatin pharmacokinetics since some juice inhibits CYP450 such as orange and grapefruit while others induces it like Liquorice.The CYP3A4 isoenzymes activity both in the intestine and in the liver is highly variable among individuals [24]. The bioavailability of Atorvastatin is low about (14%) [25, 26] and the drugs with low bioavailability are exposed to variation in drug plasma concentration. These facts may provide a reasonable explanation of high variability in Atorvastatin plasma concentrations found in this study. Liquorice have a different actions or mechanisms on the breaking down of drugs taken with Liquorice by liver [27-30]. Also there are some limitations for this study that it has been conducted on only one species and on small number of animals. Therefore, further investigations in humans are necessary to develop our findings.Abbreviation: HPLC, High Performance Liquid Chromatography. Temp, temperature. FA, Formic Acid. MRM, Multiple Reaction Monitoring. Q, Quadruple mass analyzer. DP, De-clustering Potential. EP, Entrance Potential. CEP, Collision Empotance Potential. CE, Collision Energy. CXP, Collision cell Exiting Potential. MS, Mass Spectrometric. CUR, Curtain Gas. GS, Ion Source Gas. ISV, Ion Spray Voltage.
5. Conclusions
- An analytical method with high resolution and sensitivity was used for validation and determination of Atorvastatin in rat's plasma. The effect of single and multiple doses of fresh Liquorice beverage on the pharmacokinetics profile of Atorvastatin was investigate din comparison with distilled water-feed rats. Plasma Atorvastatin levels was insignificant slightly affected by concomitant administration of either single or repeated doses of fresh Liquorice juice. The results showed insignificant elevation effect of single dose pre-treatment with Liquorice juice on the pharmacokinetic profile of Atorvastatin. In addition, the repeated dose administration of Liquorice juice showed insignificant declining in the pharmacokinetic parameters.A significant effect of single dose of Liquorice was pronounced after 2 hours from administration of Atorvastatin, and also serum levels showed a significant effect of repeated doses of Liquorice after 6 hours from administration of Atorvastatin.We can conclude from the previous results that the effects of Liquorice beverage on Atorvastatin varies between single and repeated doses and this can be a result of; either due to Liquorice different behavior or mechanisms on drugs breaking down that taken with Liquorice by liver or because of the CYP3A4 isoenzymes activity both in the intestine and in the liver which is highly variable among individuals. As the bioavailability of Atorvastatin is low about (14%) and drugs with low bioavailability are exposed to variation in their plasma concentration, this may provide a reasonable explanation of the high inter-animal variability in Atorvastatin plasma concentrations found in this study. On the other hand, there are different enteric metabolic enzymes involved in the orally administered drug metabolism. Liquorice juice could affect Atorvastatin-mediated enteric metabolic enzymes other than CYP3A4. Also CYP3A4 isoenzymes in both intestine and liver are highly variable among individuals [31, 32].Therefore, we cannot confirm these findings unless further in vitro and in vivo investigations are established. This study can leads to many possible future studies like using large number of animals, larger sample size, more than one species, and studying the effect of this combination on human plasma.
ACKNOWLEDGMENTS
- The authors wish to thank gratefully the contributors who help to complete this study in University of Petra, Amman-Jordan and Jordan Center for Pharmaceutical Research –Amman-Jordan.
Declaration
- Conflict of interestThe Authors declare that they have no conflicts to disclose.
Funding
- This research had not received specific donation from any commercial, funding agency in the public, or profit sectors. It was cooperation research from University of Petra (UOP) and Jordan Center for Pharmaceutical Research (JCPR).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML