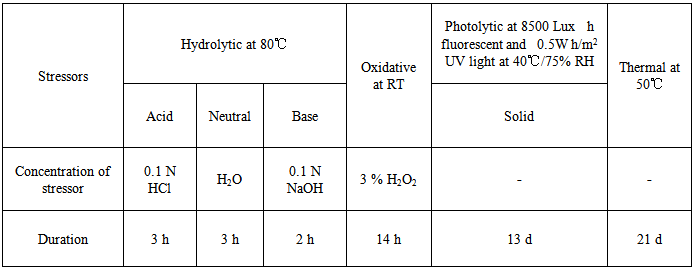
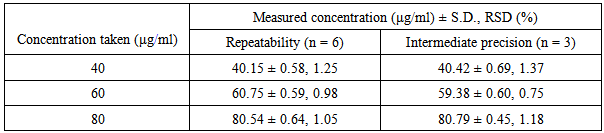
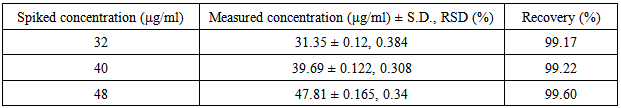
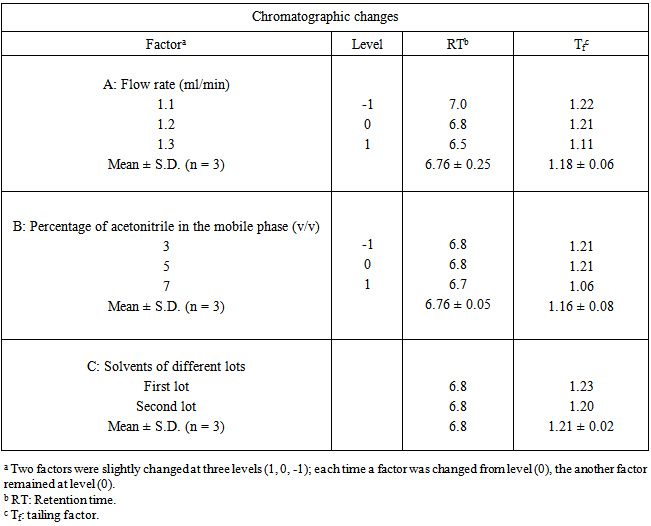
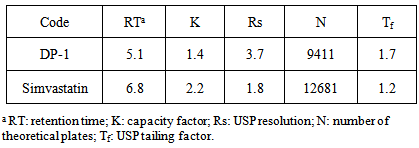
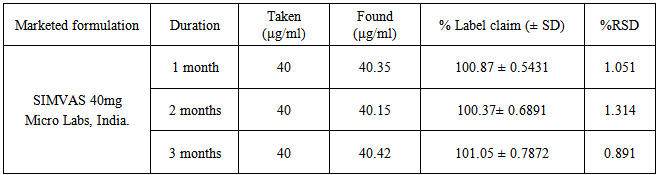
| [1] | International Conference on Harmonization (ICH), Q1A (R2): Stability testing of new drug substances and products, IFPMA, Geneva, 2003. |
| [2] | World Health Organization (WHO): Draft stability testing of active pharmaceutical ingredients and pharmaceutical products, Geneva, 2007. |
| [3] | Committee for Proprietary Medicinal Products (CPMP): Note for guidance on stability testing: stability testing of existing active substances and related finished products, EMEA, London, 2002. |
| [4] | Therapeutic Products Directorate (TPD): Guidance for industry stability testing of existing drug substances and products, Health Canada, Ottawa, ON, 2003. |
| [5] | M. Bakshi and S. Singh, 2002, J. Pharm. Biomed. Anal., 28, 1011. |
| [6] | Y. Wu, Biomed. 2000, Chromatogr., 14, 384. |
| [7] | K. D. Tripathi, Essentials of Medical Pharmacology (6 th Ed.), Jaypee Brothers Medical Publications, New Delhi (India), 2008, pp 615. |
| [8] | John H. Block and John M. Beale, Wilson and Gisvold’s textbook of Medicinal and Pharmaceutical Chemistry (11 th Edn.) Lippincott Williams and Wilkins, Philadelphia (USA), 2004, pp 662-63. |
| [9] | Chavhan V; Reddy K; Ahhirao K., 2014, Development of UV Spectrophotometric Methods and Validation for Estimation of SMV in bulk and Tablet Dosage Form by Absorbance Maxima and Area under the Curve method. J App. Pharm. 6(1), 55-64. |
| [10] | Chavhan V; Naghbhidkar N; Shukla M; Singh V. 2014UV Spectrophotometric Method Development and Validation for estimation of SMV in bulk and tablet dosage form using Mixed Hydrotropy solubilisation technique. Inter. J. Adv. Pharma. Sci. 5(1), 1740-1750. |
| [11] | Chavhan V; Lokhande S; Warimani SC; Bapat G; Sayed Mustafa., 2014, UV Spectrophotometric method for simultaneous estimation of Sitagliptin Phosphate and SMV in bulk and dosage form by Dual wavelength method. Inter. J. Pharma. Integrated Life Sci. 2(2), 1-18. |
| [12] | Arayne M. S., Sultana N., Hussain F. & Ali S. A., 2007, Validated spectrophotometric method for quantitative determination of SMV in pharmaceutical formulations and human serum. J. Anal. Chem. 62(6), 536-541. |
| [13] | Jain N; Jain R; Swami H; Pandey S; Jain DK., 2009, Spectrophotometric method for simultaneous estimation of SMV and Ezetimibe in bulk drug and its combined dosage form. Inter. J. Pharma. Pharma. Sci. 1(1), 170-175. |
| [14] | Rajput SJ; Raj HA., 2007, Simultaneous spectrophotometric estimation of Ezetimibe and SMV in tablet dosage forms. Indian J. Pharma Sci. 69(6), 759. |
| [15] | Balaji S; Sunitha A., 2010, Development and validation of spectrophotometric method for simultaneous determination of SMV and Ezetimibe in tablet formulations. Pakistan J. Pharm. Sci. 23(4), 375-378. |
| [16] | Mane VB; Babar S; Kulkarni N., 2011, Development of UV Spectrophotometric method for the simultaneous estimation of SMV and Ezetimibe in tablet dosage form by simultaneous Equation and Absorbance ratio method. Development, 3(3), 1459-1466. |
| [17] | Bhatia NM; Deshmukh DD; Kokil SU; Bhatia MS., 2009, Simultaneous spectrophotometric estimation of SMV and Ezetimibe in tablet formulation. J. Chem, 6(2), 541-544. |
| [18] | Singla V; Bhaskar R; Bhaskar R., 2003, Simultaneous Estimation of SMV and Metformin Hydrochloride in Bulk and Solid Dosage Forms. Rasayan Journal, 3, 507-513. |
| [19] | Palabiyik IM; Onur F; Yardimci C; Özaltin N., 2008, Simultaneous spectrophotometric determination of Ezetimibe and SMV in pharmaceutical preparations using chemometric techniques. Química Nova, 31(5), 1121-1124. |
| [20] | Thamake SL, Jadhav SD, & Pishawikar SA., 2009, Development and validation of method for simultaneous estimation of Atorvastatin Calcium and Ramipril from capsule dosage form by first order derivative spectroscopy. Asian J. Res. Chem. 2(1), 52-53. |
| [21] | Moussa BA; Mohamed MF; Youssef NF., 2010, Derivative spectrophotometric method for simultaneous determination of Ezetimibe and SMV in combined tablets. European J Chem. 1(4), 348-351. |
| [22] | Joshi HV; Patel JK; Kothapalli L., 2010, Simultaneous derivative and multicomponent spectrophotometric determination of SMV and Ezetimibe in tablets. Der Pharma Chemica. 2(2), 152-156. |
| [23] | Phaneemdra D; Venkatesh V; Ramarao N. 2012, Simultaneous estimation of SMV and sitagliptin by using different analytical methods. Inter. J Adv. Pharma. Anal, 2(1), 19-23. |
| [24] | Bonde P; Sharma S; Kourav N; Attar AM., 2010, Development and validated UV spectrophotometric and RP-HPLC methods for the estimation of SMV and Ezetimibe in combined pharmaceutical dosage form. Inter. J Curr. Trends Sci Tech, 1(3), 135. |
| [25] | Sharma S., 2012, Development of UV Spectrophotometry and RP HPLC Method and Its Validation for Simultaneous Estimation of Sitagliptin Phosphate and SMV in Marketed Formulation. Inter J Pharma. Biol. Arch. 3(3), 673-678. |
| [26] | Gupta A; Devu S Srinivasan KS; Gupta RS; Semwal VP., 2012, Development and Validation of Stability Indicating RP-UPLC Method for Simultaneous Determination in Fixed Dose Combination of Ezetimibe and SMV. J. Chroma. Sep. Tech. 3, 131-133. |
| [27] | Lakka NS; Goswami N; Balakrishna, P; Sailaja, V., 2011 Development and validation of a stability-indicating RP-HPLC for the simultaneous determination of Atorvastatin Calcium and SMV in pharmaceutical solid dosage forms. Int. J Res Pharm Sci, 2, 608-615. |
| [28] | Kavitha KY, Geetha G, Hariprasad R, Venkatnarayana R, Subramanian G., 2012, Development and validation of stbility indicating RP- HPLC method for the simultaneous estimation of Sitagliptin phosphate and SMV. Inter. Res J. Pharma. 3(12), 123-127. |
| [29] | Chaudhari BG; Patel NM; Shah PB., 2007, Stability-indicating reversed-phase liquid chromatographic method for simultaneous determination of SMV and Ezetimibe from their combination drug products. J. AOAC Inter. 90(5), 1242-1249 |
| [30] | L. Guzik, W. Mrozik and W. Kamysz., 2004, Determination of SMV in Pharmaceutical Dosage Forms by optimized and validated method using HPLC/UV. Chrot. Chem. Acta. 83(4), 371-377. |
| [31] | Jain N, Jain R, Swami H, Jain DK., 2008, RP-HPLC Method for Simultaneous Estimation of SMV and Ezetimibe in Bulk Drug and its Combined Dosage Form. Asian J. Res. Chem. 1(1), 29-31. |
| [32] | Shivshanker K, Sreekanth N, Harikrishnan N, Roosewelt C, Rao GS, Gunasekaran V., 2007, Validated simultaneous estimation of SMV and Ezetimibe by RP-HPLC in pure and pharmaceutical dosage form. Asian J. Chem. 19(6), 4303-4308. |
| [33] | Samaa JR., Kalakuntlab RR, Rao VS, Reddannaa P., 2010, Simultaneous estimation of SMV and Ezetimibe in pharmaceutical formulations by RP-HPLC method. J. Pharm. Sci. Res. 2(2), 82-89. |
| [34] | Hefnawy M, Al-Omar M, Julkhuf S., 2009, Rapid and sensitive simultaneous determination of Ezetimibe and SMV from their combination drug products by monolithic silica high-performance liquid chromatographic column. J. Pharm. Biomed Anal. 50(3), 527-534. |
| [35] | Madan J, Thakkar V, Dwivedi AK, Singh S., 2007, Ion-pairing RP-HPLC analytical methods for simultaneous estimation of SMV and its hydroxyl acid. J. Sci. Indust. Res. 66, 371-376. |
| [36] | Nagaraju P., 2010, A Validated Reverse Phase HPLC Method for the Simultaneous estimation of SMV and Ezetimibe in Pharmaceutical dosage forms. J Global Pharm. Tech. 2(4). |
| [37] | Kumar DA, Sujan DP, Vijayasree V, Rao JV., 2008, Simultaneous determination of SMV and Ezetimibe in tablets by HPLC. J. Chem. 6(2), 541-544 |
| [38] | Sultana N, Saeed Arayne M, Naz Shah S, Shafi N, Naveed S., 2010, Simultaneous determination of prazosin, atorvastatin, Ezetimibe and SMV in API, dosage formulations and human serum by RP-HPLC. J. Chinese Chem. Soc. 57(6), 1286. |
| [39] | Rahman MU, Parveen G, Nyola NK, Khan S, Talegaonkar S, Yar MS, Khar RK.,2010, Simultaneous estimation of SMV and Ezetimibe in pharmaceutical tablet dosage forms by RP-HPLC: A review. Int. J. Pharm. Res. Dev. 2(9), 8. |
| [40] | Neelima B, Kumar PR, Krishna MM, Bindu VH, Prasad YR., 2008Simultaneous estimation of SMV and Ezetimibe by RP-HPLC in pure and pharmaceutical dosage form. Orient. J Chem. 24(1), 195-200. |
| [41] | Dixit RP. Barhate, C. R., & Nagarsenker MS., 2008, Stability-indicating HPTLC method for simultaneous determination of Ezetimibe and SMV. Chromatographia 67(1-2), 101-107. |
| [42] | Chaudhari BG., Patel NM. & Shah PB., 2007, Determination of SMV, pravastatin sodium and Ezetimibe calcium in tablet dosage forms by HPTLC. Indian J Pharm Sci. 69(1), 130. |
| [43] | Dhaneshwar SS; Deshpande P; Patil M; Vadnerkar G; Dhaneshwar SR., 2010 Development and validation of a method for simultaneous Densitometric analysis of SMV and Ezetimibe as the bulk drugs and in the tablet dosage form. Acta Chromatogr. 20(1), 71-79. |
| [44] | Rathinaraj S., 2010, Development and Validation of A HPTLC Method for the Estimation of SMV and Ezetimibe. Inter. J. Pharma. Biol. Arch. 1(4). |
| [45] | Rathod Sonali; Patil Pallavi; Chopade Vittal. , 2012, Development and Validation of HPTLC method for the estimation of Sitagliptin Phosphate and SMV in bulk and Marketed Formulation, Int. J. Drug Dev. Res. 4(3), 292-297. |
| [46] | Barrett B; Huclova J; Bořek-Dohalský V; Němec B. & Jelinek I., 2006, Validated HPLC–MS/MS method for simultaneous determination of SMV and SMV hydroxy acid in human plasma. J. Pharma. Biomed. Anal. 41, 517-526. |
| [47] | Khaled M. Alakhali. , 2013, Method Validation for Analysis of SMV in Human Plasma Using Liquid Chromatography Tandem Mass Spectrometry (LC-MS-MS), J. Clin. Dign. Res, 7(12), 2739-43. |
| [48] | Alejandro Álvarez-Lueje, Christian Valenzuela, Juan Arturo Squella, Andluis Joaqu N N Ez-Vergara. Stability Study of SMV under Hydrolytic Conditions Assessed by Liquid Chromatography. 1631-36. |
| [49] | Wang, Jing, Shijia Liu, and Wenzheng Ju., 2007, Determination of SMV in human plasma by LC/MS/MS. China Pharma. 5, 013. |
| [50] | Tamer A. Ahmed, Jamie Horn, John Haysli, Markos Leggas, 2012, Validated LC–MS/MS method for simultaneous determination of SIM and its acid form in human plasma and cell lysate: Pharmacokinetic application. J. Pharma. Anal. 2(6), 403-11. |
| [51] | Amy Y. Yang, Li Sun, Donald G. Musson, Jamie J. Zhao., 2005Application of a novel ultralow Elution volume 96-well solid-phase extraction method to the LC/MS/MS Determination of SMV and SMV acid in human plasma, J. Pharma. Biomed. Anal. 38(3), 521. |
| [52] | Haitao Yang, Yan Feng, Yiwen Luan., 2003, Determination of SMV in human plasma by liquid chromatography–mass spectrometry, Journal of Chromatography B,785(2), 369-375. |
| [53] | Mohammed Jemal, Zheng Ouyang, Mark L. Powell, 2000, Direct-injection LC–MS–MS method for high-throughput simultaneous quantitation of SMV and SMV acid in human plasma, J. Pharma. Biomed. Anal. 23(2-3) 323-340. |
| [54] | United States Pharmacopoeia, USP30-NF25, Pharmacopeial Forum. 2002, 32(1), 3179. |
| [55] | European Pharmacopoeia. 4th Edition, Council of Europe, Strasburg Cedex. 2002. |
| [56] | S. Singh and M. Bakshi, Pharm. Tech. on-line, 1, 2000. |
| [57] | International conference on Harmonization (ICH), Q2 (R1): Validation of Analytical Procedures- Text and Methodology, Geneva, Switzerland, 2005. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML