-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2015; 5(1): 1-7
doi:10.5923/j.aac.20150501.01
Food Properties and Amino Acid Composition of Celosia spicata Leaves
Ogungbenle H. N. , Otemuyiwa F. F.
Department of Chemistry, Faculty of Science, Ekiti State University, Ado-Ekiti, Nigeria
Correspondence to: Ogungbenle H. N. , Department of Chemistry, Faculty of Science, Ekiti State University, Ado-Ekiti, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The proximate, functional properties, anti nutrients and amino acid composition of Celosia spicata leaves were investigated. The sample contained moisture content (8.16±0.01%), total ash (15.3±0.63%), crude protein (10.2±0.01%), crude fat (1.15±0.01%), crude fibre (12.3±0.02%) and carbohydrate (47.6±0.63%). The sample contained oil absorption capacity (303%), water absorption capacity (440%), emulsion capacity (56%), emulsion stability (29%) and foaming capacity (12.5%) while the least gelation concentration was 8%W/V. The water and oil absorption capacities were relatively good, and the in-vitro protein digestibility (IVPD) was 82.2%. Glutamic acid (12.8g/100g crude protein) and aspartic acid (8.96g/100g crude protein) were the most abundant amino acid while cysteine (0.40g/100g crude protein) was the least. The total essential amino acid of the sample was calculated to be 94.97g/100g crude protein which showed that the sample contained moderate quantity of essential amino acids. The sample contained tannin (97.42mg/100g), saponin (4.93mg/100g), alkaloid (0.36mg/100g), phytate (21.08mg/100g) and oxalate (16.53mg/100g) respectively. This study would provide information on the nutritional potentials of Celocia spicata leaves.
Keywords: Proximate, Functional, Anti nutrients, Amino acid
Cite this paper: Ogungbenle H. N. , Otemuyiwa F. F. , Food Properties and Amino Acid Composition of Celosia spicata Leaves, Advances in Analytical Chemistry, Vol. 5 No. 1, 2015, pp. 1-7. doi: 10.5923/j.aac.20150501.01.
Article Outline
1. Introduction
- Vegetable is an edible plant in which its parts are intended for cooking or eating raw [1]. Some vegetables can be consumed raw, while some must be squeezed severally with water and cooked to destroy certain natural toxins so as to make them edible. A wide variety of leafy vegetables are consumed in Nigeria and their consumption depends on a number of factors such as texture, nutrient and price. Vegetables play a significant role in balanced human diets, and the recommended daily requirements of minerals and vitamins are met through their consumption. The cultivation and consumption of green leafy vegetables cut across different races because of their nutritional and health benefits [2]. Vegetables contain generally low fat and protein [3, 4], varying degrees of vitamins, minerals, phytochemicals, anti oxidants, anti carcinogenic agents and fibre for gastrointestinal functions [5, 6]. Celosia specie is a vegetable grown widely in tropical Africa and also a leading vegetable in South-west Nigeria, where it is known as “sokoyokoto” meaning ‘make husbands fat and happy’. Celosia specie is an ornamental and fascinated plant probably originated from India [7]. The seedling of Celosia specie emerges 5-7days after sowing and flowering may occur in 6-7weeks aftersowing. The improved cultivars have more rapid early vegetative development but flowering occurs in 12-14 weeks after sowing. The early flowering local cultivars or wild types can be harvested only once by uprooting, but are less attractive to farmers as well as consumers, whereas the improved cultivars can be harvested by uprooting and repeated cutting of the tender vegetative parts. Celosia specie is susceptible to leaf fungi, which can be severe when air humidity is high during rainy season, thereby leading to production of poor quality leaves. The seeds and the leaves are used for medicinal purposes [8] while the soft leaves as food [9] and as livestock feed.Some previous works had been done on the other species of Celosia such as Celosia argentea, but there is scanty nutritional information on the present sample specie. Therefore, the objective of the research work is to determine the proximate, functional properties, anti-nutritional factors and amino acid of Celosia spicata leaves.
2. Materials and Methods
2.1. Sample Collection
- The fresh green leaves of Celosia spicata were obtained from a Market in Ibadan, Oyo State, South west Nigeria in Africa, during the month of February, 2014. The vegetable leaves were obtained from waterlogged area because of its scarcity during the peak of dry season.
2.2. Preparation of Sample
- The leaves were detached from the stalks, properly washed in clean water, then chopped into smaller sizes and sun-dried for 7days. The sun-dried leaves were later blended using Marlex blender into flour, then packaged in air-tight polyethylene bag and kept in freezer (-4℃) prior laboratory analyses.
2.3. Determination of Proximate Composition
- The moisture was determined using air-oven at temperature of 105℃ for 1 hr while the ash content was analyzed using a muffle furnace at 550℃ for 6 hrs [10]. The sample was analyzed for crude fat and crude protein according to the methods described by AOAC [11]. The crude fibre was determined by adding 2g of the sample into 500mL conical flask; 200mL of boiling 1.25% H2SO4 was added and boiled for 30minutes. The mixture was filtered through muslin cloth and rinsed with hot distilled water. The sample was scrapped back into the flask and 200mL of boiling 1.25% NaOH was added and allowed to boil again for another 30 minutes; filtered and then rinsed with 10% HCl twice with industrial methylated spirit and allowed to drain and dry. The residue was scrapped into a crucible, dried in the oven at 105℃, allowed to cool in a desicator and weighed; then placed in muffle furnace at 300℃ for 30 minutes and finally allowed to cool at room temperature and weighed again [11]. The carbohydrate content was calculated by difference.
2.4. Determination of Functional Properties
- The water and oil absorption capacities of the sample were determined using the method of Beuchat [12]. 10mL of water was added to 1.0g sample in a centrifuge tube. The suspension was mixed vigorously using vortex mixer. This was then centrifuged at 15,000 rpm for 15 minutes and the volume of the supernatant left after centrifuging was noted. Water bound was calculated from the difference in the initial volume of the solvent used and the final volume after centrifuging. The same procedure was used for oil absorption capacity by replacing oil with water in above process. Emulsion was prepared according to method described [13, 14]. A 2.0g sample flour was weighed with 100mL distilled water and blended for 30 sec. using Kenwood food mixer at a high speed. After complete dispersion, vegetable oil of density 0.880g per mL was added in 5mL portions from a burette with continuous blending until the emulsion break point (i.e. a separation into two layers) was observed. Emulsion capacity and stability determinations were carried out at 25oC and the value obtained was expressed as gram of oil emulsified by 1 gram sample.The emulsion stability was determined as the amount of the water separated after 24 hours at room temperature. The slight modified procedure of [15] was used to determine least gelation concentration. Sample slurries of 2, 4, 6, 8, 10, 12, 14, 16, 18, 20% were prepared in 5mL of distilled water. The test tubes containing these slurries were heated for one hour in boiling water followed by rapid cooling 2hours at 4ºC. The least gelation concentration was determined as concentration which did not slip when the test tubes were inverted. The method of Coffman and Garcia [16] was employed to determine foaming capacity and stability. 1g of the sample was whipped with 50mL distilled water for 5 minutes in a Kenwood blender and later poured into a 100mL graduated flask to study the foaming stability.
2.5. Determination of Anti Nutrients
- Oxalate: A 1g of the sample was taken into 100mL conical flask, 75mL of 1.5M H2SO4 was added and the mixture was stirred for 1 hour and then filtered. 25mL of sample filtrate was titrated against 0.1MKMnO4 solution until faint colour persisted for 30 seconds [17].Tannin: A 0.2g of the sample was added to 10mL 70% aqueous acetone and covered well. The mixture was put in an ice bath and shaken for 2hours at 30℃ and later centrifuged at 3,600rpm; 0.2mL of the mixture was pipetted into test tubes and 0.8mL of distilled water was added. Standard tannic acid solutions were prepared from a 0.5mg/mL stock and the solution made up to 1mL with distilled water. 0.5mL Folin Wu reagent was added to both sample and standard solution and then followed by the addition of 2.5mL 20% Na2CO3. The solutions were then vigorously mixed and allowed to incubate for 40minutes at room temperature after which absorbance was measured at 725nm [18].Alkaloid: The alkaloid extraction was carried out by following the modified method of Ngounou et al [19]. One gram of the sample flour was macerated (soaked) in 10mL n-hexane for about 72hours. The extract was filtered and the residue was air-dried, later treated with 10% aqueous ammonia (NH3) and soaked in trichloromethane (CHCl3) for 24hours. After the filtration and evaporation at reduced pressure, the residue was treated with 5% 7.5mL aqueous hydrochloric acid. The aqueous phase was made alkaline with aqueous NH3 and extracted three times with CHCl3. The extracted CHCl3 fraction was washed with water and the extract was transferred into a round bottom flask of Rotavapor (BÜCHI, Rotavapor R110) and separated. The concentrated extract was free of water with the use of anhydrous sodium sulphate before injected into the gas chromatography.Phytate was determined on Spectronic 20 colorimeter (Gallenkamp, UK). The amount of phytate in the sample was determined as hexaphosphate equivalent using the method of Harland and Oberleas [20]. Saponin: The extraction was carried out by following the modified method of AOAC [21]. The sample was pulverized and the saponin was extracted three times with redistilled methanol. The saponin was removed with 20mL of the solvent for 20minutes, and the combined extracts were concentrated to syrup under reduced pressure; then suspended in water. The mixture was filtered and concentrated to 1mL and 1.0μL of the extract was injected into the port of the gas chromatogram.
2.6. Determination of In-Vitro Protein Digestibility (IVPD)
- The IVPD of the sample was determined by following the modified procedure of Hsu et al. [22]. The enzymes used were trypsin, chymotrypsin and peptidase. The activity of the enzymes was initially determined by using them to digest casein. Protein isolated from the sample was ground into fine powder and sieved. The sample was dissolved in 50mL distilled water to give sample suspension of 6.25mg protein/mL. Each sample suspension was adjusted to pH 8.0 and incubated in water bath at 37OC with constant stirring. Fresh multi enzyme solution was prepared to contain 1.6mg trypsin, 3.1mg chymotrypsin, 1.4mg peptidase and dissolved in 1mL distilled water. The pH of enzyme solution was maintained at 8.0. The multi enzyme solution (5mL) was added to the sample suspension with constant stirring at 37℃. The pH of the sample suspension was recorded at 10 and 15 minutes respectively after the addition of the enzyme solution.
2.7. Determination of Amino Acid
- The amino acid profile was determined using the method described by Spackman et. al. [23]. The sample was dried to constant weight and defatted using Soxhlet extractor. After the defatting process, the defatted sample (2g) was weighed into a glass ampoule; 7mL of 6MHCl was added and oxygen was expelled by passing nitrogen into the glass ampoule sealed with bunsen burner flame and placed in an oven present at 105±5ºC for 22 hours. The ampoule was allowed to cool before broken at the tip and the content was filtered to remove the organic matters. The filterate was then evaporated to dryness at 40ºC under vacuum in a rotavapor. The residue was dissolved in 5ml of acetate buffer (pH 2.0) and stored in specimen bottles which were kept in the freezer. The hydrolysate (7.5µL) was dispensed into the cartridge of the Technicon Sequential Multi-Analyser (TSM) using a syringe. The TSM analyser is designed to separate and analyse neutral, acidic and basic amino acids of hydrolysate. The amount of amino acid was obtained from the chromatogram peaks. The whole analysis lasted for 76 minutes and the gas flow rate was 0.50mL per minute at 60ºC with reproducibility consistent within ±3%.
3. Results and Discussion
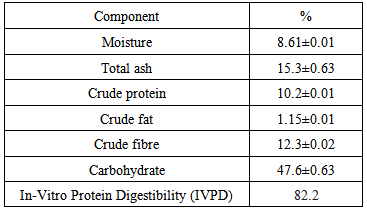
3.1. Proximate Composition
- Table 1 contains the result of proximate analysis of Celosia spicata leaves. The ash content of any food substance gives an idea of the mineral present in the food. The total ash content was lower than that for Amarathus hybridus L. (16.10%) [24], but higher than those Entada gigas (0.50%) [25], Talinum triangulare (15.10%) [24] and Alchornea cordifolia (11.38%) [26]. The total ash content was fairly high. The intake of the vegetable could be expected to contribute a large proportion of the mineral requirements in the body.
|
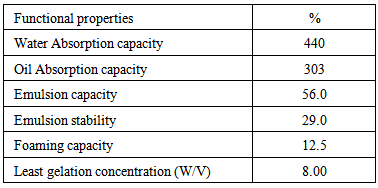
4. Functional Properties
- The functional properties of sample leaves are shown in Table 2. The water absorption capacity (440%) was lower than those of date palm fruit (45.0%) [47], soy flour (160%) [48] and lima bean (142.2%) [49]. It indicates that the Celosia spicata leaves can be used in the formulation of soups and processed cheese. The oil absorption capacity (303%) was lower than that of Afzelia africana (588.49%) [50], but higher than those values obtained for African nut meg (256%) [44], sesame protein concentrate (25.70%) [51] and conophor nut (108.13%) [52]. It is an indication that the Celocia spicata leaves can act as flavour retainer and improve the mouth feels of foods [53].
|
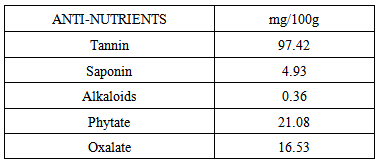
4.1. Anti Nutritional Factors
- The anti-nutritional components of Celosia spicata leaves are shown in Table 3. The presence of anti-nutritional factors in the sample is of significant importance since they have some dangerous effects on both human and animal. Oxalate has negative influence on mineral availability. The value of oxalate (16.53mg/100g) was higher than those values obtained for raw Vigna unguiculata seeds (0.40 mg/100g) [58] and cooked walnut flour (1.13mg/100g) [59]. The level of oxalate in the sample may not pose any health risk on the consumers. The phytate content (21.08mg/100g) of the sample was lower than those reported on Dannielleo oliveri kernel seed (51.05mg/100g) [60], Amarathus hybridus L. (122.5mg/100g) [61] and Ipomea batatas [62]. The problem of phytate in foods is that it can bind essential mineral nutrients in human digestive tract, resulting to mineral deficiencies [63]. The phytate composition of the sample may not pose any health but if a diet containing phytate of 10 - 60mg/100g is consumed over a long period of time may decrease bioavailability of minerals in monogastric animals [64]. The result also revealed the presence of appreciable amount of saponin (4.93mg/100g) and alkaloid (0.36mg / 100g). The value of saponin in Celosia spicata leaves was higher than that of Amarathus hybridus L. (8.5mg/100g) [61].
|
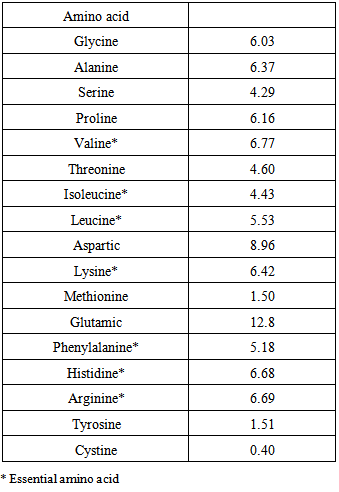
4.2. Amino acid Composition
- The amino acid composition of the sample is shown in Table 4. The result showed that glutamic acid (12.77g/100g crude protein) was the major abundant amino acid. The value was lower than those reported for Amarathus hybridus L. (15.79g/100g crude protein) [34], velvet tamarind (14.8g/100g crude protein) [28] and Moringa Oleifera leaves (8.22g/100g crude protein), but higher than stem (1.77g/100g crude protein), root (1.93g/100g crude protein) of Moringa Oleifera [29]. It is worth noting that glutamic and aspartic acids were the most abundant amino acid in agricultural products. Similar observations were also made for some legumes [34, 68, 69]. Cystine was the least amino acid in the sample, and this value was higher than those of Bidens pilosa leaf meal (0.289g/100g crude protein) [70] and Amarathus hybridus L. (0.46g/100g crude protein) [61]. The value of histidine was higher than those of maize (0.2 g/100g crude protein) [71] and gourd seed (0.21g/100g crude protein) [72]. Histidine is an essential amino acid for growth and development of infants. The methionine content (1.50g/100g crude protein) was higher than those of Bidens pilosa leaf meal (0.54g/100g crude protein) [70], and leaves of Moringa oleifera (0.99g/100g crude protein) [29], but lower than that of A. Africana (13.8g/100g crude protein) [50]. Methionine is required for choline synthesis and also may hinder fat deposition in liver [73]. Comparison between the amino acid profile of Celosia spicata leaves and the amino acid reference values reported by FAO/WHO/UNU [74] indicates that most of the amino acids in the sample meet the range of amino acid requirements recommended for infants, pre-school children and school going children as well as adults [75]. Both the histidine and arginine are essential for children [74]. Therefore, the present results indicate that the sample is a good source of quality protein and essential amino acids.
|
5. Conclusions
- The study showed that the sample is a valuable source of nutrients and essential amino acid; it is comparable to many protein rich crops, hence, it could be an alternative protein source for human consumption especially where protein sources from animal production are expensive. Celosia spicata has a high fibre content, very low crude fat, fairly high total ash which could indicate large proportion of minerals. The presence of alkaloid, tannin and saponin make the plant useful for therapeutic purposes. Other anti-nutrients such as oxalate and phytate are not too high to pose any health risk on the consumers.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML