-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2014; 4(2): 35-39
doi:10.5923/j.aac.20140402.03
Physico Chemical and Fatty Acid Composition of Nicker Bean (Entada gigas) Seed Oil
Ogungbenle H. N., OmodaraO. P.
Department of Chemistry, Ekiti State University, Ado - Ekiti, Nigeria
Correspondence to: Ogungbenle H. N., Department of Chemistry, Ekiti State University, Ado - Ekiti, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The proximate, minerals, physico chemical properties and fatty acid composition of nicker bean (Entadagigas) seed oil were evaluated. The results of proximate analysis were: moisture (25.0%), ash (0.50%), crude protein (0.23%), crude fat (60.4%) and carbohydrate (13.9%). The predominant mineral was magnesium (161mg/kg) followed by potassium (110 mg/kg) and sodium took the third position with value of 89.0 mg/kg. The least abundant mineral was phosphorus (5.45 mg/kg). The physico chemical parameters analysed were: saponification value (236mgKOH/g), specific gravity (0.7499 g/cm3), refractive index (1.4651), acid value (3.37mgKOH/g), unsaponifiable matter (21.62%), peroxide value (4.00 MEq.O2 /Kg), iodine value (13.96 mgI2/100g) and viscosity (5.52 mPa/sec). The richest fatty acid was oleic acid with the value of 72.1% while the least was lauric acid (0.11%). The value of TUFA >TSFA makes the oil worthwhile.
Keywords: Proximate, Minerals, Physico chemical, Fatty acid, Nicker, Oil
Cite this paper: Ogungbenle H. N., OmodaraO. P., Physico Chemical and Fatty Acid Composition of Nicker Bean (Entada gigas) Seed Oil, Advances in Analytical Chemistry, Vol. 4 No. 2, 2014, pp. 35-39. doi: 10.5923/j.aac.20140402.03.
Article Outline
1. Introduction
- Some of the under developed countries are dearth of food supply, especially that of protein [1]. Legumes are increasingly used by densely populated regions of the world to circumvent the precarious situation of protein shortage in diets. There is inadequate information on the nutritional parameters of some under utilised legumes. Entada gigas is commonly known as sea heart. It is a legume flowering plant of the pea family, Fabaceae. A native of central America, Caribbean and Africa. It measures 12cm (4.7inch) across and can reach 2m (6.6ft) in length. Inside the pods are 10 – 15 seeds, each of which has a diameter of 6cm (2.4inch) and a thickness of 2cm (0.70inch). The seeds contain a hollow cavity which gives them buoyancy and resistance to decay when they are washed by rain into rivers and oceans. The seeds of Entada gigas can drift long distance on ocean currents. Many legume species contain nitrogen fixing bacterial inside the root nodules which convert inert atmospheric nitrogen into ammonia, thereby making viral nitrogen available to other plants for nutrients. The pod may be 3 – 6ft long before it breaks apart into 15 or more seeded compartment, each bearing a shining brown sea heart [2]. Entada gigas has not been used by people for food due to the fact that it contains toxins which are anti nutrients but it is a very popular seed used in the preparation of traditional concoction for curing diseases in Nigeria. Some similar studies had been done by previous workers on some under utilised legumes such as Terminalia catappa [3], kidney bean [4], benniseed [5], Luffa cylindrica [6], bambara groundnut [7] and velvet tamarind [8]. The aim of this work is to determine the nutritional potentials of both flour and oil of Entada gigas.
2. Materials and Methods
- The Entada gigas was obtained from Ado - Ekiti, Ekiti State, South west Nigeria in Africa continent. The seeds were dehulled by breaking the seed coat from the inner seed using hammer. The inner seeds were further removed, dried and milled into flour using Marlex grinder. The flour was packaged in a polythene bag and kept in freezer at -4℃ prior to experimental determinations. The oil used for the analyses was extracted by using soxhlet apparatus [9].
2.1. Proximate Analysis
- The moisture and ash contents were determined using the air oven and dry ashing method [9]. The sample was analyzed for crude fat and crude protein according to the method described [10]. Nitrogen was determined by micro-Kjedahl method described [3] and the percentage nitrogen was converted to crude protein by multiplying by 6.25. The crude fibre was determined by adding 2g (W1) of the sample into 500ml conical flask; 200ml of boiling 1.25% of H2SO4 was added and boiled for 30minutes. The mixture was filtered through muslin cloth and rinsed with hot distilled water. The sample was scrapped back into the flask and 200ml of boiling 1.25% NaOH was added and allowed to boil again for another 30 minutes; filtered and then rinsed with 10% HCl twice with industrial methylated spirit and allowed to drain and dry. The residue was scrapped into a crucible, dried in the oven at 105℃, allowed to cool in a desicator and weighed (W2); then placed in muffle furnace at 300℃ for 30 minutes and finally allowed to cool at room temperature and weighed again (W3) [10].
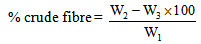 | (1) |
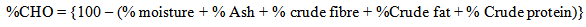 | (2) |
2.2. Mineral Analysis
- The minerals were analyzed by dry ashing the sample at 550℃ to constant weight and dissolving the ash in 100 ml standard flask using distilled deionized water with 3ml of 3M HCl. Sodium and potassium were determined by using a flame photometer (model 405, corning, U.K). All other minerals were determined by Atomic Absorption Spectrophotometer (Perkin & Elmer model 403, USA) [11].
2.3. Physico Chemical Properties
2.3.1. Determination of Saponification Value
- A 2.0ml of the oil sample was added to the 20ml of ethanolic potassium hydroxide in 500ml round bottom flask. The flask with its content was refluxed for 30 minutes. 2ml of phenolphthalein indicator was added and the hot solution was allowed to cool and later titrated against the 0.5M hydrochloric acid. A blank titration was carried out using the same procedure [9, 12].
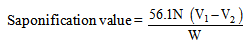 | (3) |
2.3.2. Determination of Peroxide Value
- A 2.0g of the oil sample was weighed into the 200ml conical flask containing 20ml of petroleum ether and heated for 30 seconds in a water bath. 20ml of 50% aqueous solution of potassium iodide and 25ml of distilled water were added. The resulting mixture was titrated with 0.002M sodium thiosulphate solution. During the titration a milky white precipitate was observed and the total disappearance of the precipitate indicated the end point of the titration. The peroxide value of the sample oil was estimated on the basis of the equation below. The same procedure was repeated for the blank [8].
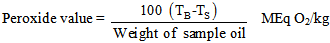 | (4) |
2.3.3. Determination of Acid Value
- A 5g of the sample oil was weighed into a 250 ml conical flask. 50 ml of hot neutralized alcohol was measured into the flask. The content in the flask was boiled on a water bath, after which 5 drops of phenolphthalein indicator was added into the content of the flask. The mixture was then titrated with 0.1M sodium hydroxide using a burette until a pink colour was observed, indicating the end point [8].
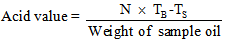 | (5) |
2.3.4. Determination of Iodine Value
- 0.2g of the sample oil was transferred into a flask containing 10ml carbon tetrachloride. 25ml of Wijs solution was added into the flask containing the sample (Wijs solution consists of iodine monochloride in glacial acetic acid). Blank was prepared. The mixture was stored in a dark place for 30 minutes at temperature of 25℃ after which 15ml potassium iodine solution was added along with 100ml of distilled water. The resulting mixture was titrated with 0.1M sodium thiosulphate solution using 2ml of 1% starch indicator. The titration was continued until the blue colour just disappeared, indicating the end point [8, 9].The iodine value was calculated on the basis of the following equation:
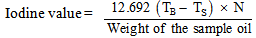 | (6) |
2.3.5. Determination of Unsaponifiable Matter
- After saponification, 300ml of the mixed solvent of ethanol (70%), toluene (25%) and 5ml oil was added to the packed glass column. It was allowed to run through the column at the rate of 12ml / minute. The glass column was washed with 150ml of the solvent mixture at the same rate. It was concentrated to 25ml using rotary evaporator and then transferred to the tarred dish for evaporation in oven at 105℃ for 15 minutes. The dried sample was weighed and titrated for the remaining acids; the weight was corrected for the unsaponifiable matter [10].
2.3.6. Determination of Specific Gravity
- The sample (40ml) was homogenized and poured into a 500ml measuring cylinder gently to avoid air bubbles. The temperature was controlled to avoid drifting in the temperature value. Hydrometer was dipped into the oil carefully to avoid resting on the wall of the cylinder and the reading was then taken [8].
2.3.7. Determination of Refractive Index
- The oil was dried to make it free of moisture. Two drops of the oil was put on the lower prism of the equipment and the prism was closed up. The water was passed through the jacket at 45℃, the jacket was adjusted until the equipment read temperature of 40℃. The light was adjusted and the compensator was moved until a dark border line was observed on the cross wire. The reading on the equipment was recorded [8].
2.3.8. Determination of Kinematic Viscosity
- A viscometer was employed for the determination. The water bath was maintained at the operating temperature by thermo regulator. The wide capillary viscometer tube was selected for the analysis. The sample was filtered through the no 200 mesh sieve to remove the associated solid particles. Sample was introduced into the viscometer in a manner stated by the equipment manual. The charged viscometer was allowed to stay in the water bath long enough to reach the test temperature. Suction was used to adjust the head level of the test sample to a position in the capillary arm of the first timing mark. As the sample was flowing freely, measurement was done to 0.2 second and the time required for the meniscus to pass from the first time mark to the second was noted. The kinematic viscosity was calculated based on equation 7 below [3].
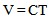 | (7) |
2.4. Fatty Acid Profile
- The fatty acid profile was determined using a method described [13]. The fatty esters analyzed using a PYE Unicam 304 gas chromatography fitted with a flame ionization detector and PYE Unicam computing integrator. Helium was used as carrier gas. The column initial temperature was 150℃ rising at 5℃ min-1 to a final temperature of 200℃ respectively. The peaks were identified by comparison with those of standard fatty acid methyl esters.
3. Results and Discussion
- Table 1 shows the result of the proximate composition of Entada gigas seed flour. The moisture content was 25.0%. This value was higher than those of quinoa flour (11.02%) reported [12], ginger bread plum seed flour (10.00%) reported [14] and velvet tamarind (8.22%) [15] but lower than stem (67.2%), root (60.7%) and leaves (75.8%) of Moringa oleifera [16]. The crude protein content (0.23%) was lower than those cereal - millet (11.4%) reported [17], ginger bread plum seed flour [14], raw African mango seed (10.6%) [11] and Luffa cylindrica (43.1%) [6]. The crude fat of Entada gigas seed (60.4%) was higher than those of ginger bread plum seed (47.28%) reported [14], bambara groundnut (6.72%) reported [7], pearl millet (7.6%) [17], T. Occidentalis (54.4%) [18], benniseed (44.3%) and bulma cotton seed (47.8%) [5].This result indicates that Entada gigas seed can be grouped as an oil-rich leguminous seed since the oil yield was 60.4%. The ash content (0.50%) was low. This value was lower than those of quinoa flour (1.2%) reported [12] and Terminalia cattapa (4.2%) [3]. While the carbohydrate content (13.9%) was lower than those of quinoa flour (58.3%) [12], Terminalia catappa (16.02%) [3] and kidney bean (40.0%) [4] but comparable with that of Luffa cylindrica (13.6%) [6]. Since the oil was high, therefore, the carbohydrate level would be very low.
|
|
|
4. Conclusions
- From the results of the analyses, it can be concluded that Entada gigas seed has a high minerals density which are essential ingredients for body development. The high oil yield and high degree of unsaturation qualify its usefulness in the industrial manufacture of pharmaceutics, soaps and cosmetics. Though the toxicity level was not reported in this paper but previous investigations revealed that the sample contains some toxins which hinder people from consuming it.
References
| [1] | Ogungbenle H.N., 2008, Nutritional quality and amino acid composition of date palm fruit (Phoenix dactilifera L) flour. Riv. Italia Sos. Grasse. 85(1):54-59. |
| [2] | Irvine, Richard, West African Crops, 3rd edition. London. Oxford University Press, 1969. |
| [3] | Nzikou, J.M., MvoulaTsieri, N.P.G. PambouTobi, C.B. Ndangui, A Kimbongui, Th. Silou, M. Linder, J. Scher and S. Desobry, 2010, Proximate composition and physic chemical characteristics of seed and seed oil from Terminalia catappa L. and the kinetic of degradation of the oil during heating. Austr. J.Basic Appl. Sci. 4, 2039-2047. |
| [4] | Olaofe, O., Famurewa, J.A.V. Ekwagbere, A.O., 2010, Chemical functional properties of kidney bean seed flour. Int. J. Chem. Sci. 3, 51-69. |
| [5] | Ogungbenle, H.N., 2006, Chemical Composition, functional properties and amino acids composition of some edible seeds. Riv. Italia Sos.Grasse.83(2);81-85. |
| [6] | Olaofe, O., Yinka, O., M.O. Aremu, 2008, Chemical evaluation of the nutritive value of smooth Luffa cylindrica seed’s kernel. Electronic Journal of Food Chemistry, 7, 3444 -3452. |
| [7] | Aremu, M.O., 2006, A comparative study on the chemical and amino acid composition of some Nigeria under utilized legume flour. Pak. J. Nutr., 5.34. |
| [8] | Ogungbenle, H. N., 2014, Sugar, physico chemical properties and fatty acid composition of velvet tamarind pulp and oil. Euro. J. Biotech. and Bioscience, 2(3), 33-37. |
| [9] | Pearson, D., Chemical analysis of foods, 7th edn. Churchill Livingstone, London, 1976. |
| [10] | AOAC., Official methods of analysis, 18th ed. Association of Official Analytical Chemists, Washington DC, 2005. |
| [11] | Ogungbenle, H.N., 2014, Chemical and amino acid composition of raw and defatted African mango kernel. British Biotechnology Journal,4(3) 244-253. |
| [12] | Ogungbenle, H.N., 2003, Nutritional evaluation and functional properties of quinoa (Chenopodium quinoa) flour. Int. J. Food Sci. and Nutr., 54,153-158. |
| [13] | Hall, G.M., 1982, Silage from tropical fishes: Lipid behavior, J. Food Technol., 21, 45-54. |
| [14] | Tidjani Amza, Issoufou Amadou, Mohammed T-Kamara, Kexue Zhu (2010). Chemical and nutrient analysis on gingerbread plum seeds (Neocarya macrophylla). Advance Journal Food and Tech. 2(4):191-195. |
| [15] | Ogungbenle, H.N. and Ebadan, P., 2014, Nutritional qualities and amino acid profile of velvet tamarind pulp. Bri. Biomedical Bulletin, 2, 006-060. |
| [16] | Olaofe, O., Adeyeye, E.I., Ojugbo, S., 2013, Comparative study of proximate amino acids and fatty acids of Moringa oleifera tree. Elixir Applied Chemistry 54, 12543 – 12554. |
| [17] | Oshodi, A.A., Ogungbenle, H.N. and Oladimeji, M.O., 1999, The Chemical composition and nutritionally valuable minerals of benniseed, pearl millet and quinoa flours. Int. J. Food Sci. and Nutri., 50, 325 – 331. |
| [18] | Olaofe, O., Adeyemi O.F and Adediran, G.O., 1994, Amino acid and mineral composition and functional properties of oil seeds. J.Agric. Food Chem. 42, 878-884. |
| [19] | Awadalkareem, A.M., Mustafa, A.I., Tinay, A.H.E., 2008, Protein, mineral and amino acid profile of sorghum flour as influenced by soy bean protein concentrate supplementation. Pak. J. Nutr., 7: 475-479. |
| [20] | Radha C, P.R Kumar and V. Prakash, 2007, Preparation and characterization of protein hydrolysates from an oil seed flour mixture. Food Chemistry, 106(3):1166-1174. |
| [21] | Ejoh R.A, Djuikwo V.N, Gouado I., and Mbofung C.M., 2007, Effect of the method of processing and preservation on some quality parameters of three non-conventional leafy vegetables. Pak. J. Nutr. 6(2): 128-133. |
| [22] | Abayeh OJ, Aina EA, Okounghae C.O., 1998, oil content and oil quality characterization of some Nigerian oil seed. Science forum, Journal of Pure and Applied Sci.,1 (1):17-23. |
| [23] | Paul A.A. and Southgate D.A.T. McCance and Widdowson’s The composition of Foods, 4th edition. The Royal Society of Chemistry, HMSO, London, UK, 1985. |
| [24] | Eromosele, I. C., Eromosele, C. O., Akintoye, A. O., and Komolafe, T. O.,1994, Characterization of oils and chemical analysis of the Seeds of wild plants. Plant Food for Human Nutrition 46, 361-365. |
| [25] | Boethouwer, C., 1983, Trends in Chemistry and Technology lipids. J. Am. Oil Chem. Soc., 60 (2): 457-462. |
| [26] | Ogungbenle, H.N. and Omowole, B.M., 2012, Chemical, Functional and Amino acid composition of Nigerian periwinkle. Int. J. Pharm. Sci. Rev. Res. 13(2), 128-132. |
| [27] | Varro E. Tyler, Lynn R. Brandy, James E. Robbers, Pharmacognosy, 7th Edition, 1976. |
| [28] | Keys, A., Anderson, J.T. and Grange, F. Prediction of serum cholesterol response of man to changes in the diet. Lancet 2, 959-966, 1957. |
| [29] | Salunkhe, D.K., Kadam, S.S. and Chavan, J.K., CRC postharvest biotechnology of food legumes. Boca Raton, FL: CRC Press, 1985. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML