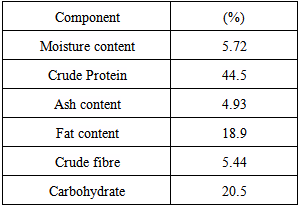
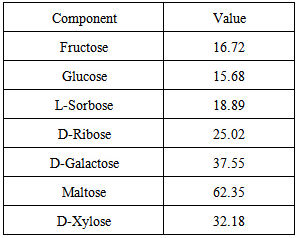
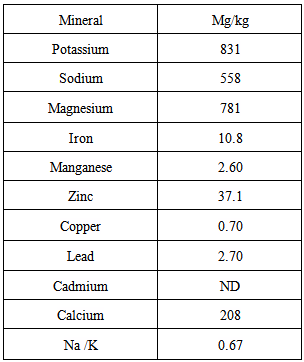
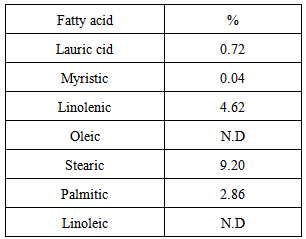
| [1] | Ukhun M.E. and Ifehigh, E.O., 1988, Compositional Chemistry of Cassia alata seeds. Food Chem. 30, 205-210. |
| [2] | Oshodi, A.A., 1992, Proximate composition, nutritionally valuable minerals and functional properties of Adenopus breviflorus benth seed flour and protein concentrate. Food Chem., 45, 79-83. |
| [3] | Ogungbenle, H.N., M. Omaejalile, 2010, Functional properties and anti-nutritional properties, in-vitro protein digestibility and amino acid composition of dehulled A. Africana seeds. Pakistan Journal of Science and Industrial Research 53, 265-270. |
| [4] | Pearson, D., Chemical analysis of foods, 7th ed. Churchill Livingstone, London,1976. |
| [5] | AOAC., Official methods of analysis, 18th ed. Association of Official Analytical Chemists, Washington DC, 2005. |
| [6] | Hall, G.M., 1982, Silage from tropical fishes: Lipid behavior, J. Food Technol., 21,45-54. |
| [7] | WHO, Energy and protein Requirement, WHO Technical Report Series No. 522. Geneva, WHO, 1973. |
| [8] | Oyenuga, V.A., Nigeria`s foods and feeding stuffs. Their chemistry and nutritive value. Ibadan Univ. Press, Ibadan, P 89, 1978. |
| [9] | Ogungbenle, H.N, 2014, Chemical, functional properties and amino acid composition of raw and defatted cashew kernel. Am. Chem. Sci. Journal, 4(3), 348-356. |
| [10] | Oshodi A.A and Adeladun MOA, 1993, Proximate composition, some valuable minerals and functional properties of three varieties of lima bean flour. Int. J.Food Sci. Nutri., 43, 181-185. |
| [11] | Oshodi, A. A. and Ekperigin, M. M., 1989, Functional properties of pigeon pea (Cajanus Cajan) flour. Food Chemistry, 34, 187-191. |
| [12] | Ogungbenle, H.N. and Omowole, B.M., 2012, Chemical, functional and amino acid composition of Nigerian periwinkle. Int. J. Pharm. Sci. Rev. Res. 13(2), 128-132. |
| [13] | Ogungbenle, H.N., 2006, Chemical composition, functional properties and amino acid composition of some edible seeds, Riv. Italia Sostanze Grasse, LXXXIII, 71-79. |
| [14] | Ogungbenle, H.N., 2003, Nutritional evaluation of quinoa flour, International Journal of Food Science and Nutrition. 54, 153-158. |
| [15] | Oshodi, A.A., Ogungbenle, H.N., Oladimeji, M.O., 1999, Chemical composition, functional properties and nutritionally valuable minerals of benniseed, pearl millet, quinoa seed flour. International Journal of Food Science Nutrition. 50, 325 – 331. |
| [16] | Aletor, V.A. and Aladetimi, O.O., 1989, Compositional evaluation of some cowpea varieties and some underutilized edible legumes in Nigeria. Die Nahrung, 33(10) 999-1007. |
| [17] | Ogungbenle, H.N., 2009, Chemical and Amino acid composition of cooked walnut (Juglans regia). Pak.J.Sci. Ind. Res. Vol.52,No.3,130-133. |
| [18] | Ogungbenle, H.N., and Adu, T., 2012, Proximate composition and functional properties of dehulled African nutmeg, Pak. J. Sci. Industrial Res.55, 80 – 85. |
| [19] | Asp N-G., 1996, Dietary carbohydrate classification and physiology. Food Chemistry 7(1):9-14 |
| [20] | Ogungbenle H.N., 2011, Chemical and Fatty acid Compositions of Date palm fruit (Phoenix dactylifera L.) flour. Bang. J.Sci.Ind.Res.46(2)255-258. |
| [21] | Brand Miller, J.C., 1994, Improvement of glycaemic index in diabetes, Am. J. Clin. Nutr., 59, 7474-7525. |
| [22] | Truswell, A.S., 1992, Glycaemic index of foods. Eur. J. Clin. Nutr., 46, S91-S101. |
| [23] | Olaofe, O. and Sanni O., 1988, Mineral Contents of Agricultural products. Food Chem. 30, 73-77. |
| [24] | Ogungbenle, H.N., Oshodi, A.A and Oladimeji, M.O., 2005, Chemical, Energy evaluation of some underutilized legume flours. Riv. Italia Sos. Grasse 82 (4) 204-208. |
| [25] | Chaney, S.G., Principles of nutrition I: Macronutrients. In: Devlin, T.M (Ed.). Textbook of biochemistry, with Clinical Correlation. 6th Edn. John Wiley and sons, New York, pp: 1071-1090, 2006. |
| [26] | Fleck, H., Introduction to nutrition 3rd Edn. New York, Macmillian, 1976. |
| [27] | Nergiz C., and Asigoz, 2008, Research on the amount of trace element in table olives commonly produced in Turkey. Riv. Italia Sostanze Grasse, LXXXV, 39-44. |
| [28] | Olaleye, A.A., Ogungbenle, H.N. and Ayeni, K.E., 2014, Mineral and fatty acid compositions of three fresh water fish samples commonly found in south western states of Nigeria. Elixir Food Science, 68, 21971-21975. |
| [29] | Paul A.A. and Southgate D.A.T., McCance and Widdowson’s The composition of Foods, 4th edition. The Royal Society of Chemistry, HMSO, London, UK, 1985. |
| [30] | Oshodi, A.A., 1996, Amino acid and Fatty acid composition of Adenopus breviflorus benth seed flour and protein concentrate. Food Chem., 45, 79-83. |
| [31] | Adeyeye, E.I, A.A. Oshodi and K.O. Ipinmoroti, K.O. , 1999, Fatty acid composition of six varieties of dehulled African yam bean flour. Int. J. Fd. Sci. and Nutr. 50, 357-365. |
| [32] | Ogungbenle, H.N. and Memenada, O.H., 2013, Physico chemical and fatty acid composition of Nigerian periwinkle. Elixir Applied Chem. 65, 19973-19975. |
| [33] | Holland, B., Unwin, I.D and Buss, H.D., Fruits and Vegetables (fifth supplement) to McCance and Widowsons. The composition of foods, Royal Society of Chemistry. London, UK, 1990. |
| [34] | Keys, A., Anderson, J.T. and Grange, F. (1957). Prediction of serum cholesterol response of man to changes in the diet. Lancet, 2, 959-966. |
| [35] | Hegsted, D.M., Calcium and Phosphorus. In Modern Nutrition in Health and Disease, ch. 6, sect. A. Lea and Febiger. Philadelphia, PA, 1993. |
| [36] | Bender, A., Meat and products in Human Nutrition in Developing Countries. FAO Food and Nutrition paper 53.Rome; FAO, 1992. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML