E. A. Basher, M. A. Z. El Tayeb
Department of Chemistry, Faculty of Science and Technology, Al-Neelain University, Khartoum, Sudan
Correspondence to: E. A. Basher, Department of Chemistry, Faculty of Science and Technology, Al-Neelain University, Khartoum, Sudan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The H- point standard addition method (HPSAM) has been applied for simultaneous determination of zinc(II) and aluminium (III) at trace levels in range 5.0×10-6 – 5.0×10-5 mol/L and 9.0×10-6-1.0×10-4 mol/L respectively, using xylenol orange (XO) a chromogenic reagent. Both Zn(II) and Al(III) form intensely red colored complex with xylenol orange at pH 4.4. The intensely red color of Zn(II) complex was developed immediately, but the color of Al(III) complex was obtained after 2 minutes. The absorbance at the selected wavelength pair, 520 and 580 nm were monitored with the addition of standard solution of Zn(II). The results showed that Zn(II)) and Al(III) ions can be determined simultaneously with concentration ratio of 20:1 and 1:20 (wt/wt). The R.S.D for a solution containing Zn(II) and Al(III) both at 1.0 x10-6 M were 2.48% and 4.41% respectively. The proposed method was successfully applied to the simultaneous determination of Zn(II) and Al(III) in synthetically spiked mixtures samples.
Keywords:
HPSAM, Zn(II), Al(III), Xylenol orange
Cite this paper: E. A. Basher, M. A. Z. El Tayeb, HــPoint Standard Addition Method for Simultaneous Determination of Zinc (II) and Aluminum (III) Ions, Advances in Analytical Chemistry, Vol. 4 No. 1, 2014, pp. 13-19. doi: 10.5923/j.aac.20140401.03.
1. Introduction
Zinc and aluminum appear together in many real samples. Zinc is an essential for normal growth, reproduction, and life expectancy of animals, [1] it is an indispensable constituent of many proteins involved in diverse cellular events such as metabolism, gene expression, and cell signaling. [2] It is essential also in plant and animal metabolism. [3] It is found in virtually all food and potable water in the form of salts or organic complexes. The diet is normally the principal source of it. [4] Aluminum derives its name from alum, the double sulfate KAl(SO4)212H2O, which was used medicinally as an astringent in ancient Greece and Rome. [5] Aluminum is white metal with many desirable properties, it is light, non toxic, capable of taking a high polish, and it is resistant to corrosion because a hard tough film of oxide is formed on the surface, [5, 6] for these reasons it has been used widely in alloys, paint, pyrotechnics and cooking utensil. [7] Several methods have been proposed for the simultaneous determination of these ions in different samples. These methods include Flame atomic absorption spectrometry [8], Cloud point extraction [9], LED photometric detection [10], Ultra sound – assisted emulsification micro extraction combined with inductively coupled plasma-optical emission spectrometry [11], etc. Among the most widely used analytical methods are those based on UV-visible spectrophotometry, due to their rapidity, simplicity and broad application.In 1988, Bosch-Reig and Campins-Falco delineated the fundamentals of H-point standard addition method (HPSAM), with which two species with mostly or even totally overlapping spectra can be determined. HPSAM is applied to work at two selected wavelengths where the analytical signal due to one of the species (interferent) is constant and for another one (analyte) to be different as much as possible. By plotting the analytical signals versus added analytical concentration, two straight lines are obtained that have a common point H with coordinates (-CH, AH), where – CH is the unknown analyte concentration and the AH is the analytical signal due to the interferent species [12]. In this work, a selective H-point standard addition method has been developed for the simultaneous determination of Zn(11) and Al(III) employing xylenol orange (XO) as a chromogenic complexing agent in a buffer at pH 4.4.
2. Experimental
2.1. Reagents
All chemicals used were of analytical reagent grade unless otherwise stated. Doubly distilled water was used throughout. A stock solution of 0.010 M Zn(II) was prepared by dissolving 0.2875 g of ZnSO4.7HO2 in 100 ml volumetric flask. A stock solution of 0.010 M Al(III) was prepared by dissolving 0.2415g of AlCl3.6HO2 in 100 ml volumetric flask. Xylenol orange stock solution of 6.5 x10-4 M was prepared by dissolving 0.125g in 0.25ml concentrated hydrochloric acid and diluted in 250 ml volumetric flask. The pH values of the working solution was adjusted using 0.20 M acetic acid and 0.20 M sodium acetate buffers. Foreign ions were prepared using their suitable salts.
2.2. Apparatus
UV-visible absorbance spectra were recorded on a Shimadzu UV-1800 scanning spectrophotometer. A Tester HANNA pH- meter using a combined glass electrode is used for measurement of the pH.
2.3. Standard Procedure
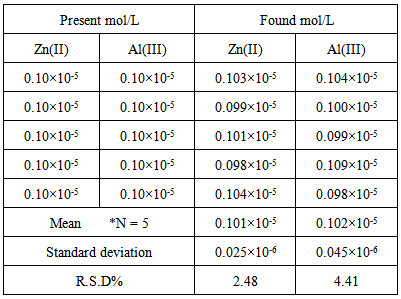
Appropriate volumes of the Zn(II) and Al(III) standard solutions, 2.50 ml buffer solution of pH 4.4 and 2.0 ml of 6.5 x10-4 M (XO) were placed in a 10.0 ml volumetric flask and volume was made up to the mark with double distilled water. The standard addition is made versus Zn(II) ion concentration, and allowed to stand for 5 min and the absorbance of solutions were measured at wavelengths 520 and 580 nm against a reagent blank. The H-point graphs are prepared by using data of absorbance and added concentration of Zn(II) ion. The concentration of Zn(II) ion is evaluated from CH. To obtain concentration of Al(III) ion, AH value and calibration curve of analytical signal versus Al(III) concentration is used to construct of this calibration curve. The absorbance measurements at wave lengths of 520 and /or 580nm were performed on samples with different concentrations of Al(III), and AH was evaluated for each sample. Five replicate samples were made to check the reproducibility of the proposed method. Results were in Table (1). Zn (II) and Al(III) were determined simultaneously using the concentration ratios of Zn(II) and Al (III) varying from 20:1 to 1:20 in mixed samples. Table 1. Results for five replicate for simultaneous determination of Zinc and Aluminum by HPSAM
 |
| |
|
Synthetic samples containing different amounts of Zn(II) and Al(III) were prepared, and the general procedure was used for analyzing Zn(II) and Al (III) content.
3. Results and Discussion
3.1. UV – VIS Absorption Spectra
As shown in Fig.(1) the absorption spectra of the colored Zn(II)-XO and Al(III)-XO complexes overlapping with each other, which prevents the simultaneous determination of these ions by conventional spectrophotometry. However, the system is amenable for simultaneous determination using HPSAM.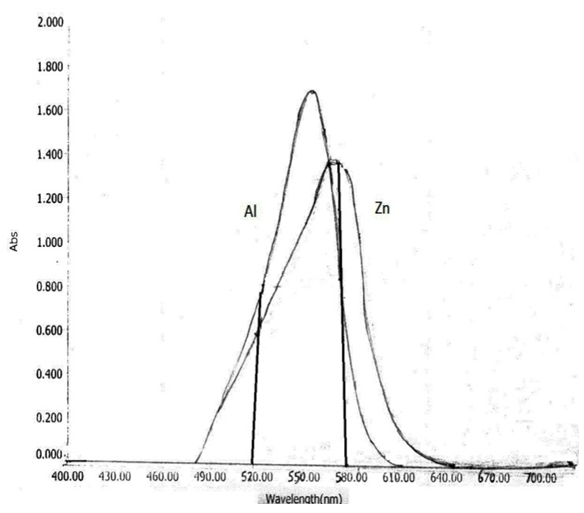 | Figure 1. Absorption spectra of 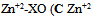 0.001M), 0.001M),  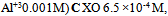 pH 4.4 pH 4.4 |
3.2. Determination of Optimal Conditions
3.2.1. Effect of pH on Complex Formation
The effect of the solution pH on the absorbencies of a constant concentration of Zn(II) and Al (III) complexes at λmax wavelengths of them was investigated in the range of pH 3-5.4 using acetic acid - sodium acetate buffer. The results from Fig.(2) showed that the pH of 4.4 gives the highest sensitivity for determination of Zn(II) and Al(III) respectively. For both cations pH 4.4 was selected as the suitable one for simultaneous analysis of Zn(II) and Al(III).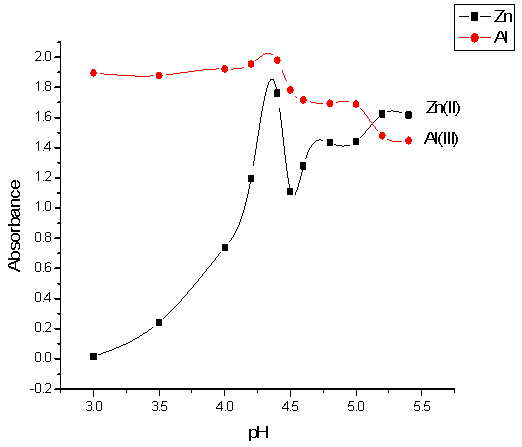 | Figure 2. Effect of pH on the absorbance of Zn-XO(■) and Al-XO(●) complexes |
3.2.2. Effect of the Volume of Buffer Solution
The effect of the volume of buffer solution on Zn(II) and Al (III) absorbencies at 572 and 554 nm λ max for each was studied in the range of 0.5 – 5 ml as shown in Fig.(3). The absorbencies of Zn+2 and Al+3 complexes increase up to 2.5ml, after which the absorbance decreases .Therefore the 2.5 ml of the volume of buffer solution was selected as the optimum volume. | Figure 3. Effect of volume of 4.4 buffer solution on the absorbance of Zn-XO(■) and Al-XO(●) complexes |
3.2.3. Effect of Xylenol Orange (XO) Concentration
The effect of different concentrations of (XO) on the absorbance of Zn(II) –XO and Al(III) – XO complexes at λmax was investigated in the range of 1.97 x 10-5M-2.9x10-4M of (XO). The results were given in Fig.(4). The absorbance increases with the increasing of (XO) concentration up to 1.3 x 10-4M and then decreasing slightly. Thus the reagent was used at concentration of 1.3 x 10-4 M.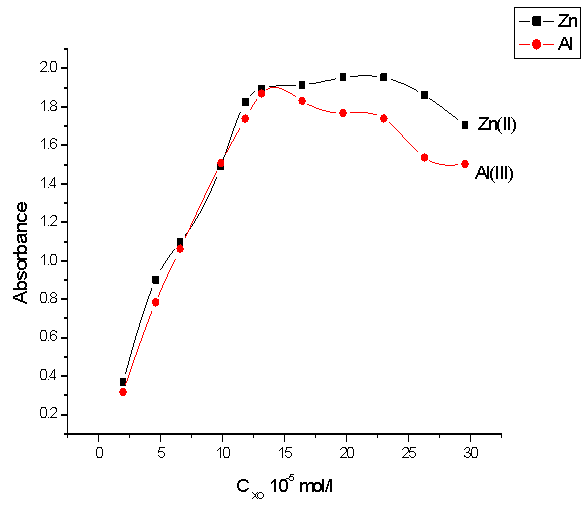 | Figure 4. Effect of xylenol orange concentration on the absorbance of Zn-XO(■) and Al-XO(●) complexes |
3.3. Individual Calibration Curves
Under the optimum conditions, calibration curves for Zn(II) and Al(III) complexes were obtained automatically by plotting the absorbance against the concentration, the curve was used to individual determination of Zn(II) and Al(III) using Beer‘s law were presented in Figs.(5) and (6). The molar absorptivites for Zn-XO and Al-XO complexes at their λ max were 145.88 and 152.34 L/mol cm3 respectively.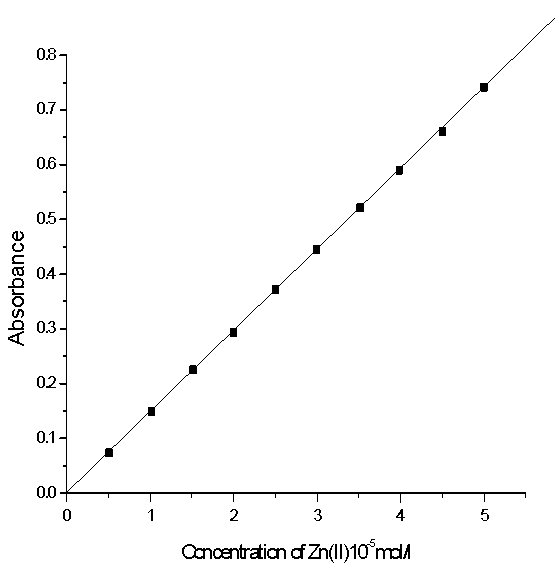 | Figure 5. Calibration curve of Zn-XO complex at 572nm |
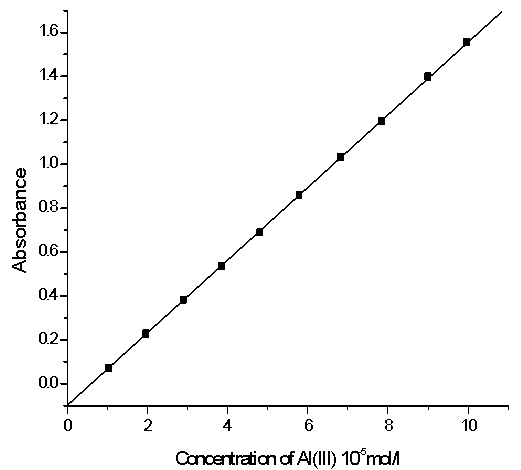 | Figure 6. Calibration curve of Al-XO complex at 554nm |
3.4. Applying HPSAM
As indicated in Fig.(1), the appropriate wavelength pair for applying HPSAM is 520 and 580 nm. The analyte signal of Zn(II) ions are linear with concentration, whereas, the interferent signal of Al (III) does not change with the increase in analyte concentration at selected wavelengths. To insure good accuracy, the Zn(II) ion is considered as analyte and Al(III) is the interferent, because the spectrum of Zn-XO complex showed a large difference in absorbances at those wavelengths.
3.5. Reproducibility of HPSAM
To examine the reproducibility of the proposed method, five replicate experiments of binary samples of Zn(II) and Al(III) ions were performed, and Table (1) presents the results. The relative standard deviations (R.S.D) were 2.48 and 4.41% for 1.0x10-6 M of each Zn(II) and Al(III) respectively.
3.6. Analysis of Zinc-Aluminum Binary Mixtures
Several synthetic mixtures with different concentration ratios of Zn(II) and Al (III) were analyzed to test the accuracy of the HPSAM for the simultaneous determination of both ions. The calibration curves at the selected wavelengths were plotted as shown in Figs.(7) and (8). The concentration of Zn(II) (analyte) and Al(III) (interferent), were determined by the ordinary values of CH and AH respectively. The results are given in Table (2).Table 2. Simultaneous determination of Zinc and Aluminum ions in binary mixtures by HPSAM
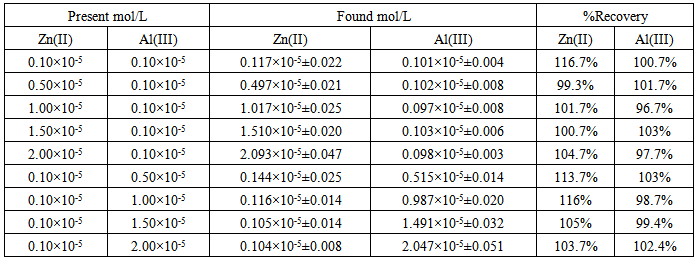 |
| |
|
Figs.7 and 8 also show the H-point standard addition plots for several synthetic test solutions with constant concentration of Zn(II) and Al (III) ions respectively. These Figures demonstrate the independent of CH on the Al(III) concentration and the independent of AH values on Zn (II) concentration. 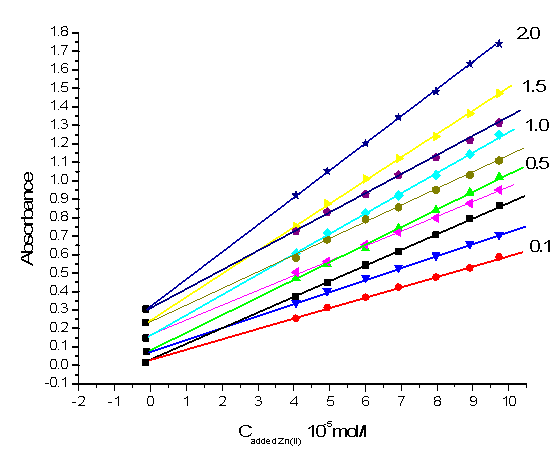 | Figure 7. H-point standard addition plot for simultaneous determination of zinc and aluminium under optimized conditions with a constant concentration of zinc (1.0 × 10-6 M) and aluminum concentration of: 1.0 × 10-6 M, 5.0 × 10-6 M, 1.0 × 10-5 M, 1.50 × 10-5 M and 2.0 × 10-5 M |
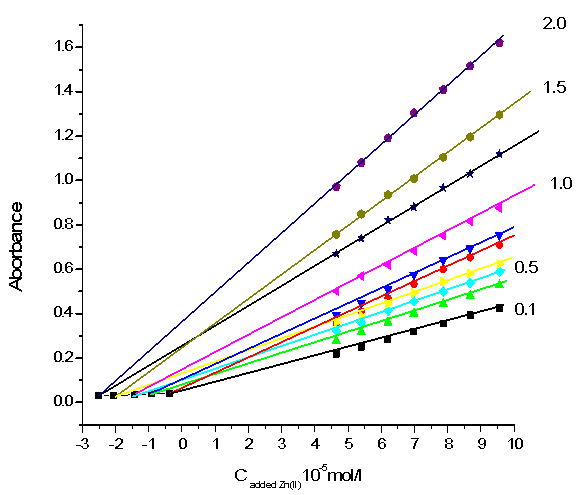 | Figure 8. H-point standard addition plot for simultaneous determination of zinc and aluminium under optimized conditions with a constant concentration of aluminum (0.10 ×10-5 M) and zinc concentration of: 1.0× 10-6M, 5.0× 10-6 M, 1.0 ×10-5 M,1.50 ×10-5 M and 2.0× 10-5 M |
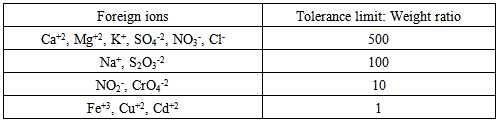
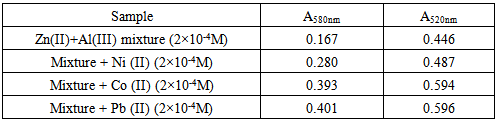
3.7. Effect of Foreign Ions
The effect of various diverse ions on the absorbance of a solution containing 2.0x10-4M each of Zn (II) and Al(III) in mixture were studied. The tolerance limit was defined as the concentration of the added ion causing less than a ± 2% relative error. The results for both ions Table (3) indicate that most of the cations and anions showed no significant interference at weight ratios greater than 1000. Table (4) shows Co(II), Pb(II) and Ni(II) gave positive effect on the signal, therefore the presence of these cations must be removed in order to obtain accurate and precise results.Table 3. Effect of foreign ions on the determination of Zn(II) and Al(III) complexes
 |
| |
|
Table 4. Effect of foreign cations on the determination of Zn(II) and Al(III) complexes
 |
| |
|
4. Application of the HPSAM
HPSAM was applied to the determination of Zn(II) and Al(III) in several real samples. For this purpose, spiked tap water, biscuits, and green tea samples were analysed. The results of analysis were summarized in Table (5). The good agreement between the obtained results and the known values indicate the successful applicability of HPSAM for simultaneous determination of Zn(II) and Al(III) in complex samples.Table 5. Determination of zinc and aluminum in different samples by HPSAM
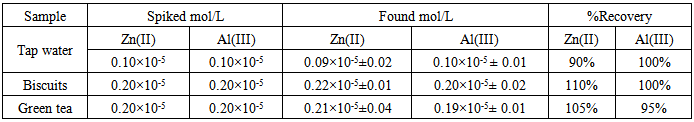 |
| |
|
5. Conclusions
In our best of knowledge this is the first simultaneous determination of zinc and aluminum by the H-point standard addition method, using xylenol orange as chromogenic reagent at pH 4.4. HPSAM provides satisfactory results in synthetic mixtures and spiked sample, this method is highly sensitive, highly selective, simple and rapid.
References
| [1] | H.A. Harper, V.W. Rodwell and P.A. Mayes, "Review of physiological chemistry" 17th Ed., Langt Medical publication, Canada, 1979, 575-595. |
| [2] | C. E. Sabel, J. L. Shepherd and S. Siemann, Analytical Biochemistry 391, 2009, 74-76. |
| [3] | J. D. Hem, "Study and Interpretation of the Chemical Characteristics of Natural Water" U.S. Geological survey, water supply paper 2254,U.S. Government printing office, Washington,1992,3-144. |
| [4] | WHO. "Guide lines for Drinking Water Quality" 2nd Ed., Vol. 2, Geneva, 1996. |
| [5] | N.N. Greenwood and A. Earnshaw "Chemistry of the elements". Pergmon Press Ltd. 1st edition 1984. |
| [6] | F.A. Cotton and G. Wilkinson "Advance inorganic Chemistry". 2ed edition, John Wiley &Sons. 1966. |
| [7] | J.D. Lee, A new concise inorganic chemistry, 3rd edition. Van Nostrand Reinhold UK Co. Ltd 1977. |
| [8] | M. Tuzen and M. Soylak, Polish J. of Environ. Stud., 15, 2006, 915-919. |
| [9] | A.B. Tabrizi, Food Chemistry, 100, 2007, 1698-1703. |
| [10] | M. Trojanowicz and J.S. Obinska, Analytica. Chimica. Acta., 230, 1990, 125-130. |
| [11] | H. Sereshti, S. Bakhtiari and V. Khoieh, Anal. Metheds, 3, 2011, 2936-2942. |
| [12] | R. Rohilla and U. Gupta, Chem. Sci. Trans., 1(3), 2012, 582-589. |

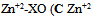 0.001M),
0.001M), 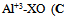
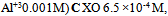 pH 4.4
pH 4.4




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





