-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2013; 3(4): 57-61
doi:10.5923/j.aac.20130304.04
Determination of Trace Elements in Particles released from Coal Combustion
Ali A. Eltayeib
Department of Chemistry, Faculty of Science, Kordofan University, Sudan
Correspondence to: Ali A. Eltayeib, Department of Chemistry, Faculty of Science, Kordofan University, Sudan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this study, the atomic spectroscopic methods for determination of some trace elements were established. ICP-AES was used for determination the trace elements Co, Ni, Cu, Cr, etc. For the laboratory research work, all the coal samples were digested with a mixture of acids (HNO3- HF- HCLO4) after burned at 650 C0 for one hour. Based on the establishment of atomic spectroscopic determination methods of trace elements, Tessier sequential speciation and separation methods were used in the study of speciation distribution of some heavy metals in different size of particles released from coal combustion of Qingshan thermoelectric plant. The transition elements in two samples from Qingshan thermoelectric plant (with different combustion condition) were extracted into five fractions by sequential extraction. In each fraction a suitable reagents with an optimum pH and time were used. Different circumstance of combustion results in different distribution of elements. The released metals increase with the decreasing of pH and the concentration of salts of leashing reagents.
Keywords: Trace Elements, Coal Combustion, Dreg, ICP-AES
Cite this paper: Ali A. Eltayeib, Determination of Trace Elements in Particles released from Coal Combustion, Advances in Analytical Chemistry, Vol. 3 No. 4, 2013, pp. 57-61. doi: 10.5923/j.aac.20130304.04.
Article Outline
1. Introduction
- Practically all the elements of the periodic table are present in coal, sometimes in the minerals, sometimes in the organic matter, or in both, depending on the coal properties and on the physico-chemical processes occurring before or after maturation of the coal. The major elements, Al, Fe, Ca, Mg, and K, are present in the coal mineral fraction whereas the trace elements Zn, Cu, Mn, Cr, Ni, Co, etc, are associated with the mineral fraction and/or the organic coal fraction. Different trace elements in coal have different distribution characters in coal combustion. The distribution and concentration of trace elements in coal are important from the environmental point of view, since they permit prediction of the behavior of these elements as they are released into the atmosphere when the coal is burned. The trace elements associated with sulfide and organic matter generally enriched in fly ash because they are known by their volatilization during coal combustion. Volatilization probably does not occur in trace elements associated with the alumino-silicate minerals. It is important to point out that the enrichment of trace elements on the ashes is also a function of the particle size. Enrichment of trace elements on the surface of fly ash, especially the submicrometer particles. This enrichment can be explained through the volatilization condensation mechanism based on the higher concentration of the trace elements in the fly ash (especially on the surface particles) emitted into the atmosphere, when compare with those collected by control equipment(ex: electrostatic precipitator). Several experimental procedures, varying in manipulative complexity have been proposed for determining the speciation of particulate trace metals. These procedures can be grouped into (1) methods designed to effect the separation between residual and non residual metals only, and (2) more elaborate methods making use of sequential extractions. The former methods normally involve a single extraction and offer a better contrast between anomalous and back ground samples than does the determination of the total metal concentration. Despite their rapidity and relative simplicity these techniques suffer from the difficulty of finding a single reagent effective in dissolving quantitatively the non residual forms of the metal without attacking the detrital forms. The use of sequential extraction although more time consuming, furnishes detailed information about the origin, mode of occurrence, biological and physicochemical availability, mobilization, and transport of trace metals. Two major experimental problems with sequential procedures have been recognized: non selectivity of extractants, and trace element redistribution among phases during extraction[1]. Generally it is difficult to associate a given extractant reagent with a particular physicochemical phase. Most validation studies have focused on selectivity and extraction completeness rather than redistribution and predominantly from the stand point of major species rather than trace elements[2-4]. The partitioning obtained by such procedures is, however, influenced by factors such as the choice of reagents used for the various extractions, the extraction sequence, and the time of extraction[2, 5-7].In this work, based on the research of the distribution of trace element in coal, Tessier sequential extraction method was used to evaluation of speciation of some trace elements in particles released from coal combustion.
2. Experimental
2.1. Instrumentation and Reagents
- A spectro ICP-AES (Made in W. Germany 1991) controlled by an IBM ps/2 computer, was used throughout this work. PH meter and centrifuge were also used. Pump speed 2ml/min and tube length 110cm. The power of emission 1200 W. All reagents were of analytical-reagent grade, and doubly de-ionized water was used throughout. The stock standard solutions used were as follows: GBW (E) 08005 100µg/ml for Cd, WB0113 100µg/ml for Co, GSBG62023-90 1000µg/ml for Cu, GBW (E) 1ooµg/ml for Cr, and GBW (E) 080007 100µg/ml for Ni.1.0 ml was taken from each stock solution of the elements Cd, Co, Cr, and Ni, and 0.1 ml from the stock solution of Cu, all they were mixed in 100 ml volumetric flask and diluted to the mark with distilled water.
2.2. Methods
2.2.1. Sampling
- The samples studied were from Qingshan thermoelectric plant, there are two types of samples according to combustion condition 170 t/h, and 220 t/h.Sample 170 t/h was burned in an oxidizing condition, temp. 1261C0, CO2 13.12%, O2 6.15%, CO 0.014%.Sample 220 t/h was burned in reducing condition, temp. 1323C0, CO2 17.76%, O2 1.29%, CO 0.12%.Two different particles (dreg) were sampled for each condition of combustion.
2.2.2. Sample Preparation
- The dreg was first grinded and 100 mesh sieve was used to separate the fine particles from the coarse particles. The samples were dried at 106C0 for one hour, and then one gram from each sample was weighed. The sample can be partitioned into specific fractions which can be extracted selectively by using appropriate reagents. For total trace metal analysis, the sample was also dried at 106C0 for one hour, and 1.0g was weighed accurately and then burned at 650C0 for one hour. The sample was digested with the following acids: 3 ml HNO3, 5 ml HF, and 5 ml HCLO4 after addition of the acid the solution was evaporated to near dryness and finally 10 ml of HCl were added until the appearance of bubbles and at last the sample was diluted to 50 ml and then analyzed by ICP-AES.
2.3. Sequential Extraction
- There are five fractions chosen which are likely to be affected by environmental conditions.(i)-Exchangeable The coal was extracted at room temperature for 1h with 8 ml of magnesium chloride solution (1 M MgCl2, pH 7.0)(ii)-Bound to carbonate The residue from (1) was leached at room temperature for 5h with 8 ml of 1M NaOAC adjusted to pH 5.0 with acetic acid. The extraction was maintained with continuous agitation.(iii)-Bound to Fe-Mn oxideThe residue from (2) was extracted with 20.0 ml of 0.3M Na2S2O3 + 0.175M Na-citrate + 0.025 M H-citrate. The experiment was performed at 96±3C with occasional agitation for 6h.(iv)-Bound to organic matterTo the residue from (3) 3 ml of 0.02 M HNO3 and 5 ml of 30% H2 O2 adjusted to pH 2.0 with HNO3 were added and the mixture was heated to 85±2C for 2h with occasional agitation. A second 3.0ml aliquot of 30% H2 O2 (pH=2 with HNO3) was then added and the sample was heated again to 85±2C for 3h with intermittent agitation. After cooling, 5.0ml of 3.2M NH4 OAC in 20% (v/v) HNO3 was added and the sample was diluted to 20.0ml and agitated continuously for 30min. the addition of NH4 OAC is designed to prevent adsorption of extracted metals onto the oxidized coal.(v)-Residual For residual trace metal analysis, the solid was digested with a 10:1 mixture of hydrofluoric and perchloric acids. The residue was first digested with a solution of concentrated HCLO4 (2ml) and HF (20) to near dryness; subsequently a second addition of HCLO4 (1ml) and HF (10ml)was made and again the mixture was evaporated to near dryness. Finally, HCLO4 (1ml) alone was added and the sample was evaporated until the appearance of white fumes. The residue was dissolved in 12N HCL and diluted to 25.0 ml.
2.4. Acid Rain Simulation
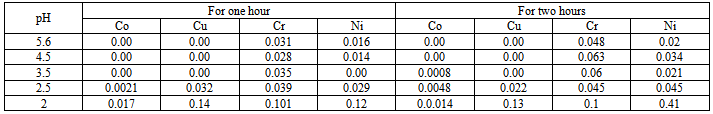
- 3.0g of 170 t/h sample of fly ash (1.7µm) was taken. 0.0173g CaCl2, 0.0111g MgSO4 and 0.0083g NH4 NO3 were dissolved in 1L volumetric flask and diluted to the mark. 300 ml was taken from the volumetric flask and divided into 5 flasks, then the pH in each flask adjusted with H2SO4, the pH is as follows: 2, 2.5, 3.5, 4.5, and 5.6.10 samples were arranged, 5 for one hour and the other 5 for two hours.30 ml was taken from each flask and added to the first 5 samples (1h), again 30 ml from each flask was taken and added to the other 5 samples (2h). The extracted solution was analyzed by ICP-AES for trace elements.
|
|
|
|
3. Results
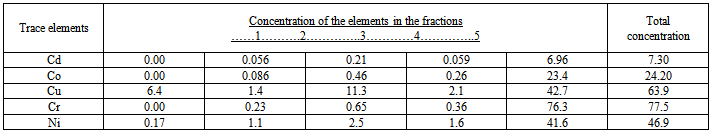
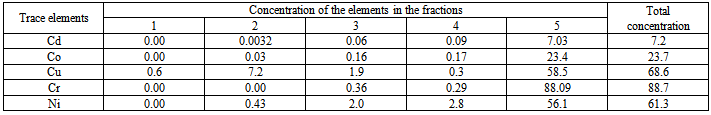
- The results of speciation by Tessier sequential extraction for dreg under different conditions of combustion are shown in tables 1, and 2.In order to evaluate the reliability of speciation method of Tessier sequential extraction, the total amount of trace elements in different samples of dreg were determined. The results are shown in table 3.The results of acid rain simulation are shown in table 4.
4. Discussion
- Many reagents, including ammonium acetate[8,9,10-13], sodium acetate[10], and magnesium chloride[14,15], have been employed to liberate exchangeable metals. Of these, NH4OAC (pH 7.0) had been the most extensively used in soil and sediment analysis. However, according to many workers[10,12,13], it may also attack carbonates. According to Chapman[10], the solubility of CaCO3 is much lower in 1M sodium acetate at pH 8.2. for this reason NH4OAC was not considered in this study. Reagents recommended for dissolving selectively the carbonate fractions in sediments generally make use of sodium acetate and /or acetic acid at acidic pH values[8,16-18]. Grossmann and Millet[19] reported that organic carbon and free iron concentrations in noncalcareous soil samples were unchanged after contact with this buffer for nine weeks; other workers[8,18] have demonstrated that lower pH values lead to partial attack of Fe and Mn oxides. The time required for carbonate dissolution will depend upon such factors as particle size, percentage and type of carbonate present, and sample size[17]. The most successful methods for leaching iron and manganese oxides involve the combined action of reagents reducing these metals to their ferrous and manganous forms, respectively, and of agents capable of keeping in solution the relatively large amounts of metals liberated; the two couples most often used are hydroxylamine hydrochloride-acetic acid [8,16] and sodium dithionite-citrate[14,8,15,17,20], with the latter combination, using concentrations prescribed by Anderson and Jenne[21], they observed substantial precipitation of trace metals, presumably due to the formation of sulfide as a result of the disproportionation of dithionite[2]. Examination of the results of Gupta and Chen[8], who employed both combinations in parallel experiments, reveals that trace metals concentrations were consistently lower in the leachates obtained with dithionite-citrate than in those obtained with hydroxylamine hydrochloride-acetic acid; the differences were greatest for those trace metals which form the most insoluble salts. Hydrogen peroxide in an acidic medium is generally used to oxidize organic matter in soil[17,13] and sediment[8] analysis, even though oxidation of all forms of organic matter may not be complete[13]. More efficient methods for destroying organic matter do exist (e.g. concentrated nitric acid used separately or in combination with hydrochloric or perchloric acids), but they usually suffer from a lack of specificity in the sense that they may also effect a partial attack of silicate lattices. A method involving treatment with hot hydrogen peroxide in a nitric acid medium was adopted[8]. The behavior of trace elements in coal combustion is partly dependent on the mode of occurrence of the element in the original coal[22-24] which has an impacts on human health and environment. Dale and Chapman[22] used sequential leaching separations in three steps: 4.3M HCl for 2h at 80C0, 0.5M HNO3 at 80C0 for 2h and 40% HF+ conc.HCl at 60-70C0 for 1h. willett and others[23] used in their sequence of separation 1N ammonium acetate.From these results it appeared that the total concentrations of the trace elements in 170 t/h dreg sample and 220 t/h dreg sample are nearly the same except for Ni and Cr which are higher in 220 t/h than 170 t/h and the extraction in each fraction depend on the temperature of combustion. The content of the exchangeable part is very low because, exchangeable species is ion species adsorbed on the surface of particles, after combustion (high temp.) the trace elements is not the adsorbed species. The content of carbonate is also low, during combustion, carbonate decompose, but some part of the metal oxide may react with CO2 to form carbonate, therefore there is small quantity of carbonate in dreg. Because of the uncompleted combustion of coal, there are small quantities of organic matter in the dreg, so there are some organic associated trace elements in the dreg. The results showed that the organic associated part are higher than exchangeable and carbonate associated part. The trace elements in this part may be release into water system under oxidation condition or biological effect. The content of Fe-Mn oxide associated trace elements are similar to those in organic matter. From these results higher temperature for combustion increase the concentration of some trace elements in the dreg and this will lead to decrease of trace elements in fine particles. Only small parts of the total trace elements release into water when act with acid rain, release quantity increase with the decrease of pH of acid rain, when pH˂ 2.5 release is significant high. The effect of the concentration of MgCl2 and NaOAC can be observe in acid rain extraction pH 4.5~5.6 and the sequential extraction pH 7 and pH 5. The extraction in sequential extraction is higher than in acid rain simulation. This shows that the release of trace elements from particles into water system not only depends on pH of leaching agent but also on the concentration of the salt in leaching agents. The sum of the five fractions equal the total determination. This shows the results of sequential extraction were reliable. The results of extraction at 2h higher than in 1h (some times lower) can be explained by sorption-desorption on the surface of particles.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML