V. Jagannadham1, R. Sanjeev2
1Department of Chemistry, Osmania University, Hyderabad-500 007, India
2Department of Chemistry Mizan-Tepi University, Tepi Campus, Tepi, Ethiopia
Correspondence to: V. Jagannadham, Department of Chemistry, Osmania University, Hyderabad-500 007, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
A simple and easy laboratory protocol for the determination of the pKa of a weak acid like hydrazoic acid (HN3) is described in this paper by measuring the absorbance values of the hydrazoic acid as a function of pH with a ‘UVIKON 323 Kontron Instruments’ Spectrophotometer. An equation compatible to KaleidaGraph program is derived and used in the program to determine the pKa. The present protocol for the determination of the pKa is useful in teaching chemistry experiments in Physical Chemistry Laboratory.
Keywords:
pKa, KaleidaGraph, Kontron Instruments, Acid-Base Equilibriums
Cite this paper: V. Jagannadham, R. Sanjeev, The KaleidaGraph and the Kontron: “Hands On” Tools in a Physical Chemistry Laboratory for Determination of pKa of Weak Acids and Weak Bases, Advances in Analytical Chemistry, Vol. 3 No. 4, 2013, pp. 54-56. doi: 10.5923/j.aac.20130304.03.
1. Introduction
The acid-base ionization/dissociation constant, pKa, is a measure of the tendency of a molecule or ion to keep a proton (H+) at its ionization center(s), and is related to the ionization ability of the acid or base. pKa is the core property of an electrolyte that defines chemical and biological behavior. In biological terms, pKa is important in determining whether a molecule will be taken up by aqueous tissue components or lipid membranes and is related to log P (where P the partition coefficient). Water is a very polar solvent (dielectric constant ε20 = 80), so facile ionization will increase the likelihood of a species to be taken up into aqueous solution. If a molecule does not readily ionize, it will tend to stay in a non-polar solvent such as cyclohexane (ε20 = 2) or octanol (ε20 = 10). Separation and analytical scientists require an understanding of pKa because it impacts the choice of techniques used to identify and isolate the compound of interest. pKa is also closely related to the concept of pH (the acidity of the solution). Hence the knowledge of pKa values is useful to the majority of the pharmaceutical companies worldwide[1]. Also knowing the acidity constant value (Ka) of an acid and its associated pKa value is important for medical students for different reasons. The first reason is that the knowledge of pKa of a drug allows predicting the absorption, bio-reactivity and tissular accumulation as a function of the pH of the medium[2]. Another reason is that the pKa values of the amino acids of a polypeptide chain are related to the function and structure of the protein[3]. In addition, the pKa values of different chemical species will help understanding the biological systems[4,5].
2. Experimental
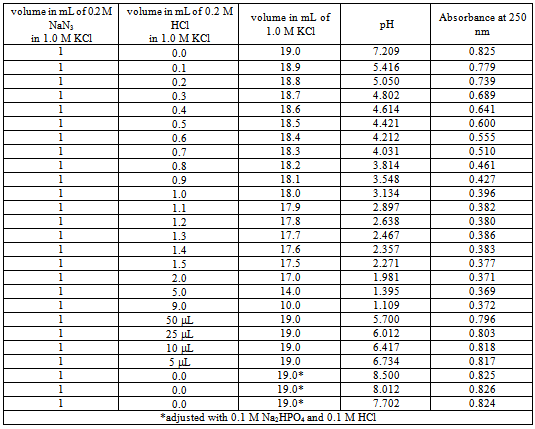
All the chemicals used were of analytical grade. All the solutions were prepared in double distilled water. The absorbance values were recorded on a ‘UVIKON 323’ Kontron Instruments Spectrophotometer imported from Italy. The software program used for the curve fitting of the absorbance versus pH was a ‘KaleidaGraph’ from Synergy Software, Reading, PA, USA. The ionic strength was maintained at 1.0 M using KCl. To obtain the absorbance of HN3 at 250 nm as a function of pH, suitable volumes of HN3, HCl and KCl are mixed as shown in the table. From each sample 3 mL of solution was taken in 3 mL quartz cuvette and the absorbance was measured. The pH meter used was from ‘Radiometer, Copenhagen’.
3. Results and Discussion
3.1. Theoretical Basis
The students must be familiar with the acid-base equilibriums. Populations of dissociated and un-dissociated species of weak acid change with pH. Consider the dissociation of a weak acid in aqueous solutionTable 1. Variation of absorbance of azide
 |
| |
|
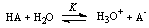 | (1) |
The equilibrium constant K could be written as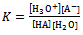 | (2) |
or 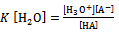 | (3) |
or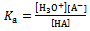 | (4) |
Where Ka is the acid dissociation constant,[H3O+] is the concentration of hydrogen ion,[A-] is concentration of salt, and[HA] is that of un-dissociated acid. Taking the logarithms of equation 4, we get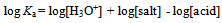 | (5) |
or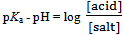 | (6) |
Equation 6 contains total of three variables, the pH which is an independent variable and[acid] and[salt] which are two different dependent variables. The two dependent variables must be transformed in to one dependent variable in terms of the absorbance of the species preferably the anion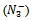 of the weak acid HN3, so that eventually the equation could best be transformed in to an equation of a locus with two variables. This is done as follows:Equation 6 is rewritten as
of the weak acid HN3, so that eventually the equation could best be transformed in to an equation of a locus with two variables. This is done as follows:Equation 6 is rewritten as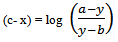 | (7) |
Where c is pKa, x is pH, y is observed absorbance which changes with pH, a and b are the average absorbance of the salt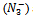 at highest pH say after the pH between 7.0 and 8.5 and average absorbance of the acid (HN3) at the lowest pH say between 1 and 2 respectively. Therefore (a - y) and (y - b) are the concentrations of the acid and the salt in terms of one variable that is ‘y’, the absorbance which changes as a function of pH. Therefore equation 7 now contains eventually only two variables x and y. And this could be further transformed in to the equation of a locus with two variables as follows:Equation 7 could be written as
at highest pH say after the pH between 7.0 and 8.5 and average absorbance of the acid (HN3) at the lowest pH say between 1 and 2 respectively. Therefore (a - y) and (y - b) are the concentrations of the acid and the salt in terms of one variable that is ‘y’, the absorbance which changes as a function of pH. Therefore equation 7 now contains eventually only two variables x and y. And this could be further transformed in to the equation of a locus with two variables as follows:Equation 7 could be written as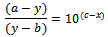 or
or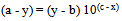 or
or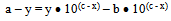 or
or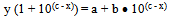 or
or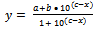 | (8) |
And this is converted to an equation which could be understood by KaleidaGraph software to calculate the pKa of HN3 and is written as: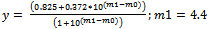 | (9) |
Where m1 = pKa and m0 = x and 0.825 is ‘a’ which is the average absorbance above the pH 7 and 0.372 is ‘b’ which is that of average below the pH 2 and m1 is arbitrarily given a value of 4.4. After several iterations the KaleidaGraph program calculates the pKa value, which came out to be 4.42 and a locus is passing through the experimental points which is a sigmoid (Figure 1)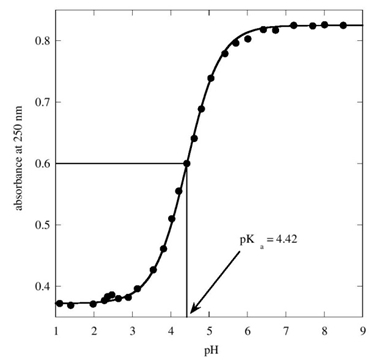 | Figure 1. Plot of absorbance versus pH |
In equation 6 the quantity on right hand side is put equal to 1 so that at half neutralization the pH = pKa. With this condition y comes out to be 0.599 using the values 0.825 and 0.372 for ‘a’ and ‘b’ respectively, which is the absorbance of the species at half neutralization. Identifying the value of 0.599 on the y-axis on the graph, proceeding to the locus perpendicularly to the y-axis and from there interpolating on to x-axis gives the pH for half neutralization which is the pKa of the acid. The literature value of pKa of HN3 is 4.7[6]. There were several methods of determination pKa values of weak acids[7].Hence, the ‘KaleidaGraph’ software and the Kontron instruments are two ‘Hands On’ tools for any graduate program laboratory.
References
| [1] | Sandra Babic´, Alka J.M. Horvat, Dragana Mutavdzˇ ic´ Pavlovic, Marija Kasˇtelan-Macan, Trends in Analytical Chemistry, Vol. 26, No. 11, 2007 |
| [2] | L. L. Brunton, J. S Lazo and K. L. Parker, “Las Bases Farmacológicas de la Terapéutica,” McGraw Hill, España, 2006. |
| [3] | L. Stryer et al., “Bioquímica,” Reverté, Barcelona, España, 2003. |
| [4] | A. C. Guyton, “Tratado de Fisiología Médica,” Elsevier, España, 2006. |
| [5] | T. Devlin, “Bioquímica,” Reverté, Barcelona, España, 2003. |
| [6] | E. P. Serjeant and B. Dempsey (eds.), Ionization Constants of Organic Acids in Solution, IUPAC Chemical Data Series No. 23, Pergamon Press, Oxford, UK, 1979. |
| [7] | http://www.raell.demon.co.uk/chem/logp/ logppka.htm |

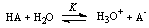
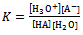
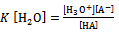
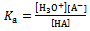
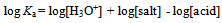
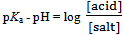
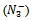 of the weak acid HN3, so that eventually the equation could best be transformed in to an equation of a locus with two variables. This is done as follows:Equation 6 is rewritten as
of the weak acid HN3, so that eventually the equation could best be transformed in to an equation of a locus with two variables. This is done as follows:Equation 6 is rewritten as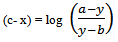
 at highest pH say after the pH between 7.0 and 8.5 and average absorbance of the acid (HN3) at the lowest pH say between 1 and 2 respectively. Therefore (a - y) and (y - b) are the concentrations of the acid and the salt in terms of one variable that is ‘y’, the absorbance which changes as a function of pH. Therefore equation 7 now contains eventually only two variables x and y. And this could be further transformed in to the equation of a locus with two variables as follows:Equation 7 could be written as
at highest pH say after the pH between 7.0 and 8.5 and average absorbance of the acid (HN3) at the lowest pH say between 1 and 2 respectively. Therefore (a - y) and (y - b) are the concentrations of the acid and the salt in terms of one variable that is ‘y’, the absorbance which changes as a function of pH. Therefore equation 7 now contains eventually only two variables x and y. And this could be further transformed in to the equation of a locus with two variables as follows:Equation 7 could be written as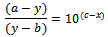 or
or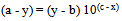 or
or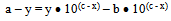 or
or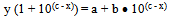 or
or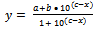
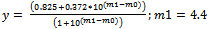

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML