-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2013; 3(3): 29-33
doi:10.5923/j.aac.20130303.02
Enantioseparation of Efavirenz by Ultra Performance Liquid Chromatography
Surendra Dutt Sharma, Gaurav Singh
Analytical Research Laboratory, School of Sciences, IFTM University Campus, Moradabad, 244102, Uttra Pradesh, India
Correspondence to: Gaurav Singh, Analytical Research Laboratory, School of Sciences, IFTM University Campus, Moradabad, 244102, Uttra Pradesh, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
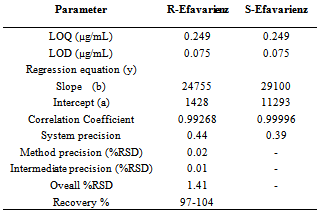
A rapid isocratic chiral UPLC method has been developed for the separation of R-Efavirenz from S- Efavirenz. Good resolution viz. Rs> 3.0 between R- and S- forms of Efavirenz was achieved by NP-UPLC using chiral column Chiracel OD-H(250 mm x 4.6 mm) 5 µm. The mobile phase was n-Hexane and Isopropyl alcohol (IPA) in 90:10 ratio (v/v). Column temperature was 30°C and flow rate was kept 1.0 mL min-1.The elution was monitored by photodiode arraydetector at λ-254 nm. The calibration curve showed excellent linearity over concentration range 0.249-375 μg mL-1. This method was further used to determine the amount of R-Efavirenz in pure and active pharmaceutical ingredient of S-Efavirenz and is capable to quantitate and detect R-Efavirenz to the levels of 0.249 μgmL-1 and 0.075 μg mL-1 respectively. The average recovery range ofR- Efavirenzwas 97-104 %.
Keywords: Enantioseparation, (R,S) -Efavirenz, UPLC, SM-FTN
Cite this paper: Surendra Dutt Sharma, Gaurav Singh, Enantioseparation of Efavirenz by Ultra Performance Liquid Chromatography, Advances in Analytical Chemistry, Vol. 3 No. 3, 2013, pp. 29-33. doi: 10.5923/j.aac.20130303.02.
Article Outline
1. Introduction
- Chirality is a major concern in the modern pharmaceutical industry. This interest can be attributed largely to a heightened awareness that enantiomers of a racemic drug may have different pharmacological activities, as well as different pharmacokinetic and pharmacodynamic effects. The body being amazingly chiral selective, will interact with each racemic drug differently and metabolize each enantiomer by a separate pathway to produce different pharmacological activity. Thus, one isomer may produce the desired therapeutic activities, while the other may be inactive or, in worst cases, produce unwanted effects[1-3]. Consideration of chirality is now an integral part of drug research, development and the regulatory process. Enantiomeric forms of a drug can differ in potency, toxicity, and behaviour in biological systems. Enantiomers of all chiral bioactive molecules have to be separated and tested. The Food and Drug Administration (FDA, U.S.A.), and regulatory authorities in Europe, China, and Japan have provided guidelines indicating that preferably only the active enantiomer of a chiral molecule should be used to formulate the doses form[4].Chiral HPLC has proven to be one of the best methods for the direct separation and analysis of enantiomers. It is more versatile than chiral GC because it can separate a wide variety of non-volatile compounds. Direct chiral separations using CSPs are more widely used and are more predictable, in mechanistic terms, than those using chiral additives in the mobile phase. HPLC has been widely used in pharmaceutical industries for the enentiomeric separation of chiral molecules e.g. Clopidogrel, Galantamine, Naproxin[5,6,7]. High performance liquid chromatography (HPLC) is a proven technique that has been used in laboratories worldwide over the past 30-plus years. One of the primary drivers for the growth of this technique has been theevolution of packing materials used to effect the separation. The underlying principles of this evolution are governed by the van Deemter equation, which is an empirical formula that describes the relationship between linear velocity (flow rate) and plate height (Height equivalent to a theoretical plate or column efficiency). Since particle size is one of the variables, a van Deemter curve can be used to investigate chromatographic performance[4,8].According to the van Deemter equation, as the particle size decreases to less than 2.5 µm, not only is there a significant gain in efficiency, but the efficiency does not diminish at increased flow rates or linear velocities of the mobile phase. By using smaller particles, speed and peak capacity (number of peaks resolved per unit time in gradient separations) can be extended many folds and termed Ultra Performance Liquid Chromatography, or UPLC. The technology takes full advantage of chromatographic principles to run separations using columns packed with smaller particles and/or higher flow rates for increased speed, with superior resolution and sensitivity[4,8].Efavirenz[(S)-6-Chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl-2H-3,1-benzoxazin-2-one]is non-nucleoside reverse transcriptase inhibitor (NNRTI) and is used as a part of highly active antiretroviral therapy for the treatment of human immunodeficiency virus (HIV) type-1[9].Immobilised polysaccharide derived chiral stationary phase are extensively applicable to separate enantiomers of large class of organic compounds[10-11]. The determination of the enantiomeric purity of the organic compound was, therefore, determined by developed diastereomer analytical method(s), using column having immobilised polysaccharide derived chiral stationary phase as a selector.A survey of literature reveals that an attempt has been made for the assay and quantification of R-Efavirenz impurity in the bulk drugby HPLC[12]. However, enantiomeric resolution ofS-Efavirenz and R-Efavirenz (Fig.1a & 1b) has not yet been carried out by UPLC.
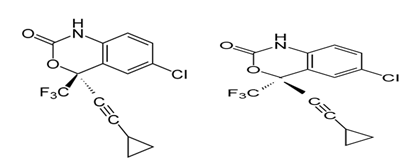 | Figure 1. a. Structure of S-Efavirenz, b. Structure of R-Efavirenz |
2. Experimental
2.1. Instrumentation
- Chromatographic analysis is performed with waters ACQUITY UPLC coupled with photodiode array detector (Compounds were detected at λ-254 nm) and column Chiracel OD-H(250 mm x 4.6 mm) 5 µm is used for chromatography. The oven temperature of UPLC was at 30°C and injection volume is 10 µL. The flow rate of the mobile phase was adjusted at 1.0 mL min-1 and the total run time is 30 min. Chromatographic data were controlled and processed on a computer running with millennium empower version no:.2.0.
2.2. Chemicals
- Efavirenz Active pharmaceutical ingredient is from Crams India Limited, Faridabad (India). N-Hexane and Isopropyl alcohol (HPLC grade) are from Merck (India).
2.3. Preparation of System Suitability Solution
- 5.0 mg of Efavirenz enantiomer and Efavirenz standard is weighed into a standard 50 mL volumetric flask.It is thoroughly dissolved with 25 mL of diluents and the volume was made up with diluent up to the mark and mixed thoroughly. Further dilute 0.25 mL of this solution to 10 mL with diluent.
2.4. Preparation of Sample Solution
- 25 mg of sample is weighed accurately and transferred into 100 mL volumetric flask. It is dissolved with 10 mL of diluents. The solution is now made up with diluents up to the mark and thoroughly mixed.
2.5. Preparation of Mobile Phase
- A mixed degassed solution ofn-Hexane and Isopropyl alcohol (IPA) in 90:10ratio (v/v) is prepared.
3. Result and Discussion
- Immobilised polysaccharide based stationary phases play a significant role in the chiral separation science. Cellulose and amylose derivatives are used as the stationary phases in the chiral columns and these chiral stationary phases have the good capability for the chiral resolution[10-15].Cellulose and amylose polysaccharides contain polymeric chains of D-(+) glucose units.These glucose units are joined through β-1,4 linkage in cellulose and α-1,4 linkages in amylose (Fig-2a & 2b). Cellulose contains glucose units which ranges from 200-14,000. Similarly, more than 1000 glucose units are found in amylose. Each glucose unit has chair conformation with 2-OH, 3-OH and 5-CH2OH groups all in equatorial position. In the cellulose the chains of glucose units lie side by side in a linear fashion whereas in amylose the glucose units lie in the helical manner[10, 15, 16].
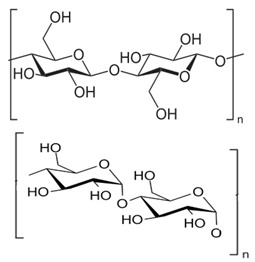 | Figure 2. a. Structure of Cellulose,2b. Structure of Amylose |
 | Figure 3. 3D structure of Cellulose, showing chiral grooves |
4. Method Validation
- After the systematic optimization, the final conditions of this method have been extensively validated for chiral separation by UPLC determination.
4.1. Precision
- The precision of the chromatographic system was checked by injecting six replicate injection of system suitability solution having the concentration 2.5 µg mL-1 of S-Efavirenz and R-Efavirenz (Fig-4).The repeatability and intermediate precision of the proposed method was determined by a six fold analysis of 250 µgmL-1ofS-Efavirenz,which is spiked with 0.20 % ofR-Efavirenz (Fig-5). The % RSD of R-Efavirenz found in the spiked samples was calculated.
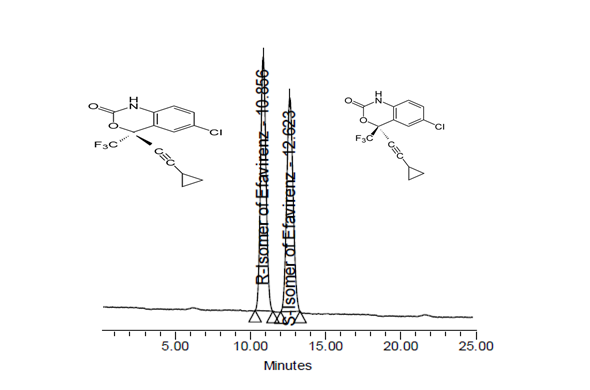 | Figure 4. Resolution solution Chromatogram |
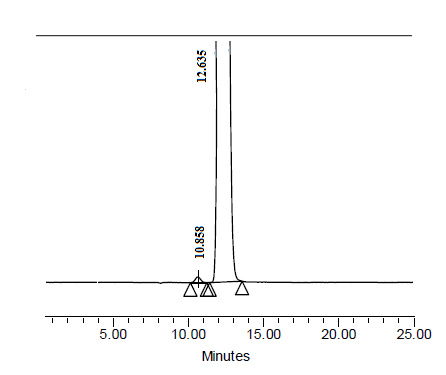 | Figure 5. Chromatogram of S-Efavirenz sample spiked with impurity of R-Efavirenz |
4.2. Limit of Detection (LOD) and Quantification (LOQ)
- The LOD and LOQ for S-Efavirenz and enantiomeric impurity R-Efavirenz were determined at a signal to noise ratio of 3:1 and 10:1respectively, by injecting a series of dilute solution with known concentration of S-Efavirenz and R-Efavirenz. Precision study was carried out at the LOD & LOQ level by injecting six replicate injections (n=6) individually and the % RSD for the area counts was calculated.
4.3. Accuracy
- The accuracy of the analytical method express the closeness of agreement between the true value and the value found by the specific analytical method which is being used for the analysis. For impurities, recovery was determined in triplicate for 0.16, 0.20 and 0.24 % of the analyte concentration (250 µg mL-1) on drug substance and recovery of the impurity was calculated.
4.4. Linearityof Response
- Linearity for R-Efavirenz and S-Efavirenz was assessed by injecting separately prepared solutions covering the range from LOQ to 150 %of the normal sample concentration (250 µg mL-1). The correlation coefficients, slopes and Y-intercept of the calibration curve were determined.
4.5. Analytical Solution Stability
- S-Efavirenz solution spiked with 0.20 % of R-Efavirenz prepared in the diluent were injected at 0 hr to 24 hr at different time intervals and the % absolute area difference of the R-Efavirenz area counts was checked.
4.6. Robustness
- The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate, variations in method parameters and provides an indication of its reliability during normal usage. To determine the robustness of the method, the experimental conditions were deliberately changed. The system suitability and spiked sample were evaluated. The mobile phase flow rate was 1.0 mL-1.To study the effect of the flow rate on resolution, the flow rate was changed to 0.9 mL-1 and 1.1 mL-1. The effect of the column temperature was studied at 28°C and 32°C (instead of 30°C), and the mobile phase composition was changed to 88:12 and 92:8 from the initial composition.
5. Validation of the Method
- The RSD (%) of peak area for R-Efavirenz and S-Efavirenz in the study of therepeatability is shown in Table-1. Results for intermediate precision (intra- and inter-day repeatability) are within 1.0%. These results confirmed that the method is highly precise.
5.1. Limits of Detection and Quantification
- The limit of detection and limit of quantification for R-Efavirenz and S-Efavirenz are reported in Table-1.
5.2. Accuracy
- It is observed that the percentage recovery of impurity R-Efavirenz in bulk drug samples ranged from 97% to 104%.
5.3. Linearity
- For R-Efavirenz and S-Efavirenz, linear calibration curve was obtained ranging from LOQ, 25%, 50%, 75%, 100%, 125%, and 150%. The correlation coefficient obtained is greater than 0.999 (Table-1). The results indicate excellent linearity.
5.4. Robustness
- In all the deliberate varied chromatographic conditions (flow rate, mobile phase composition and column temperature), all analyte peaks were adequately resolved and elution orders remain unchanged.
5.5. Stability in Solution and in the Mobile Phase
- No significant changes in the amounts of the impurity R-Efavirenz were observed during solution stability and mobile phase experiments when performed using the determination of R-Efavirenz in bulk drug method. The results obtained from solution and mobile phase stability experiments confirmed that standard solutions and sample solutions in the mobile phase were stable for up to 24 hr during determination of R-Efavirenz in bulk drug method.
|
6. Conclusions
- A simple, rapid and linear NP-UPLC method is given for the quantitative separation of R-Efavirenz from S-Efavirenz. The method is simple as it does not need any derivatization to diastereomers and economical too because of very short run time.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML