N. M. Modawe1, 2, M. A. Z. Eltayeb1
1Department of Chemistry, Faculty of Science and Technology , Al-Neelain University, Khartoum, Sudan
2Abstracted from the M. Sc. thesis of N. M. M.
Correspondence to: N. M. Modawe, Department of Chemistry, Faculty of Science and Technology , Al-Neelain University, Khartoum, Sudan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The H-point standard addition method (HPSAM) was applied for the simultaneous spectrophotometric determination of cobalt(II) and nickel(II) in range 10-5 to 10-4 mol/ L. The new synthesized 2, 4-dihydroxybenzaldehyde thiosemicarbazone (DHBATSC) was used as a chromogenic reagent in aqueous Triton X-100 micellar medium and at pH 7. The absorbance at the selected wavelength pair, 463 and 500 nm were monitored with the addition of standard solution of cobalt. The results obtained showed that Co(II) and Ni(II) can be determined simultaneously with concentration ratios of 5:1 to 1:5 (wt/wt). The R.S.D for a solution containing Co(II) and Ni(II) both at 5.0 × 10-5 M were 1.86% and 4.4% respectively. The effect of diverse ions on the determination of cobalt(II) and nickel (II) were also investigated. The proposed method was successfully applied to the simultaneous determination of cobalt(II) and nickel(II) in synthetically spiked mixtures and cobalt(II) in vitamin B12 sample.
Keywords:
H-point, Cobalt(II), Nickel(II), Spectrophotometric method, 2, 4-dihydroxy benzaldehyde thiosemicarbazone
Cite this paper: N. M. Modawe, M. A. Z. Eltayeb, H–Point Standard Addition Method for Simultaneous Spectrophotometric Determination of Cobalt(II) and Nickel(II), Advances in Analytical Chemistry, Vol. 3 No. 1, 2013, pp. 1-7. doi: 10.5923/j.aac.20130301.01.
1. Introduction
Cobalt is always found in nature in association with nickel. Biochemically influence of cobalt appears in vitamin B12, which is important in the treatment of pernicious anemia.[1] Cobalt is not considered to be as toxic as most of the heavy metals, although insufficient or excessive intake may lead to deficiency or toxicity that results in flushing, vasodilatation and cariomyopathy in humans and animals.[2]Nickel and cobalt are essential trace elements in human diet. Nickel is bound to ribonucleic acid which has been suggested to have a role in pigmentation.[3]There is a claim that certain compounds of nickel are carcinogenic. Also Ni(II) can cause allergic reactions.[4] Since nickel and cobalt appear together in most real samples both natural and artificial, several methods have been proposed for their simultaneous determination. These methods include atomic absorption spectrometry (AAS), electrothermal atomic absorption spectrometry (ETAAS) flame atomic absorption spectrometry (FAAS),[3] high performance liquid chromatography(HPLC),[4] inductively coupled plasma-atomic emission spectrophotometry (ICP-AES), neutron activation analysis (NAA) and x-ray fluorescence (XRF),[5] differential pulse polarography (DPP),[6] adsorptive cathodic stripping voltammetry, gravimetric, and spectrophotometric methods.[7-9] Among the most widely used analytical techniques are those based on uv-vis spectrophotometry, due to their simplicity and rapidity and broad application. Bosch-Reig et al outlined the fundamentals of HPSAM in 1988 which is a simple bivariate chemometric technique. HPSAM is applied to work at two selected wavelengths where the analytical signals due to one of the species (interferent) is constant and for another one (analyte) to be different as much as possible. By plotting the analytical signal versus added analyte concentration, two straight lines are obtained that have a common point with coordinates H (-CH, AH), where –CH is the unknown analyte concentration and AH the analytical signal due to interferent species [10]In this work, a precise H-point standard addition method has been developed for the simultaneous determination of cobalt(II) and nickel(II). The determination was carried out in a Triton X-100 aqueous micellar medium using a new synthesized chromogenic complexion agent at neutral pH7.
2. Experimental
2.1. Reagents
All chemicals used were reagent grade. Doubly distilled water was used throughout. A stock solution of 0.010 M Co(II) was prepared by dissolving 0.291 g of Co(NO3)2.6H2O and drops of conc. HNO3 in 100 ml volumetric flask. A stock solution 0.010 M Ni(II) was prepared by dissolving 0.297 g of Ni(NO3)2.6 H2O and drops of conc. H2SO4 in 100 ml volumetric flask. DHBATSC stock solution of 0.010 M was prepared by dissolving 0.211 g in 100 ml ethanol. Adjusting the pH values of the working solution was carried out using acetic acid-sodium acetate and phosphate buffers.
2.2. Apparatus
UV-visible absorbance spectra were recorded on a Shimadzu UV-1800 scanning spectrophotometer. A Tester HANNA pH – meter using a combined glass electrode is used for measurement of the pH.
2.3. Preparation of 2, 4-dihydroxy Benzaldehyde Thiosemicarbazone[11]
A 2,4-dihydroxy benzaldehyde(I) ( 4.144 g – 30.0 mmol) and thiosemicrbazide (II) (2.730 g-30.0 mmol) were dissolved in 100 ml ethanol (85%), the mixture was refluxed for 3 hours. The content was allowed to cool then separated by filtration. A crude sample yield (80%) was obtained. The resultant product was recrystallized with cold ethanol. Pure light yellowish crystals of 2, 4-dihydroxy benzaldehyde thiosemicarbazone (DHBATSC) (III) m.p (226℃) were obtained.
2.4. General Procedure
For the determination of Co(II) and Ni(II) ions using HPSAM, the synthetic solution containing Co(II) and Ni(II) ions at different concentration ratios were prepared with 1.0 ml buffer solution of pH 7, 2.0 ml of 2 ×10-3M (DHBATSC) and 1.0 ml Triton X-100 (10 % v/v) In a 10- ml volumetric flasks. The standard addition is made versus Co(II) ion concentration and the absorbances of solutions are measured at wavelengths 463 and 500 nm against a reagent blank .The H-point graphs are prepared by using data of absorbance and added concentration of Co(II) ion. The concentration of cobalt(II) ion is evaluated from CH. To obtain concentration of Ni(II) ion, AH value and calibration curve of analytical signal versus Ni(II) concentration is used to construct of this calibration curve, the absorbance measurements at wavelengths of 463 and/or 500 nm were performed on samples with different concentrations of Ni(II), and AH was evaluated for each sample. Five replicate samples were made to check the reproducibility of the proposed method. Results were in Table (2).Synthetic samples containing different amounts of Co(II) and Ni(II) were prepared, and the general procedure was used for analyzing Co(II) and Ni (II)content. Vitamin B12 tablets were decomposed in a 50.0 ml round-bottomed flask by heating with a mixture of concentrated nitric acid (10.0 ml) and sulfuric acid (1.0 ml) on a hot plate until near dryness. The residue was neutralized with a dilute solution of sodium hydroxide and diluted in a 25-ml volumetric flask with deionized water. The cobalt content was analyzed using the general procedure.
3. Results and Discussion
3.1. Synthesis of chromogenic reagent
The comparison of the measured melting point 226℃ of the synthesized reagent with the literature confirmed the formation of the ligand. The ligand (DHBATSC) reacts with nickel and cobalt to form yellow and brown colored complexes respectively. Maximum color development occurs in neutral to slightly acidic media. The brown color of the Co(II) complex was developed immediately, but the yellow color of the Ni(II) complex was obtained after 3 minutes.
3.2. UV-VIS Spectra of Cobalt and Nickel Complexes
As displayed in Fig. (1) the absorption spectra of the colored Co(II)-DHBATSC and Ni(II)-DHBATSC complexes show overlapping with each other, which prevents the simultaneous determination of these ions by conventional spectrophotometry. However, the system is amenable for their simultaneous determination using the H-point standard addition method.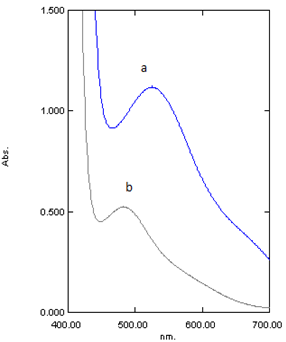 | Figure (1). Absorption spectra of (a) Co2+-DHBATSC complex (CCo 1.00×10-4M) and (b) Ni2+-DHBATSC complex (CNi 1.00×10-4M) , conditions C DHBATSC 2.50×10-3M, pH 7 in Triton X-100(10%v/v) |
3.3. Determination of Optimal Conditions
3.3.1. Effect of pH
The effect of the solution pH on the absorbencies of Ni(II) and Co(II) complexes at λmax wavelengths of them was studied in the pH range of 3.0 – 8.0 using acetic acid - sodium acetate and phosphate buffers. As shown in Fig. (2), the absorbance of both Co-DHBATSC and Ni-DHBATSC complexes increase up to pH 7, after which the absorbance decreases. Therefore the pH 7 was selected as the optimum solution. 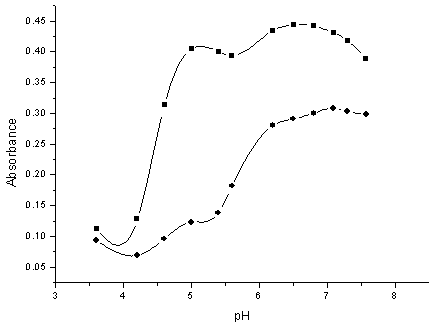 | Figure (2). Effect of pH on the absorbance of Co 2+-DHBATSC(■) complex , CCo 1.00× 10-4M at 525 nm and Ni 2+-DHBATSC(●) complex ,CNi 1.00× 10-4M at 480 nm, C DHBATSC 2.50× 10-3M |
3.3.2. Effect of DHBATSC Concentration
The effect of the concentration of the reagent (DHBATSC), for Co(II) and Ni(II), absorbances at 525 and 480 nm λmax for each was investigated in the range of 1 ×10-3M to 5× 10-3M of (DHBATSC) . The results were given in Fig. (3). The absorbance increases with the increasing of (DHBATSC) concentration up to 2×10-3 M and then decreasing slightly. Thus, (DHBATSC) was used at concentration of 2.5 ×10-3M.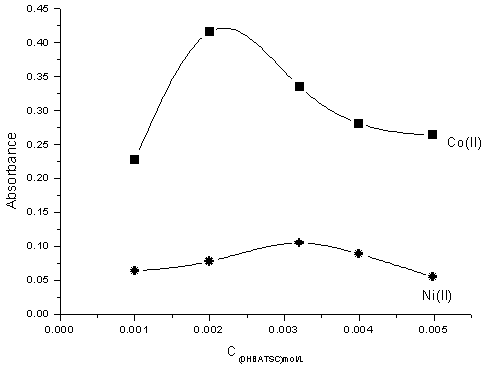 | Figure (3). Effect of DHBATSC concentration of on the absorbances of Co- DHBATSC (■)at 525nm and Ni-DHBATSC (●) 480nm,C Ni,Co 1× 10-4 M complexes, pH 7 and Triton X-100 (10%v/v) |
3.3.3. Stoichiometry of the Complexes
The stiochiometry of the Co(II) complex was carried out by Job's continues variation method. From Fig. (4), the ratio was found to be 1:2 (metal: ligand). 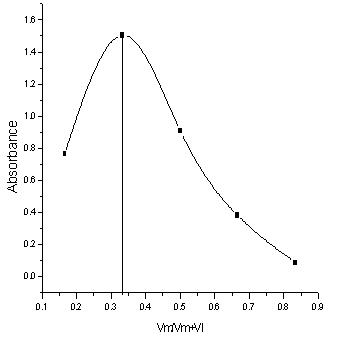 | Figure (4). Continuous variation method of Co-DHBATSC complex at 525nm, pH 7 and Triton X-100 (10%v/v) |
3.3.4. Effect of Surfactant
Calibration curves for individual determination of Ni(II) and Co(II) using Beer´s law are presented in Fig. (5). In the presence of Triton X-100, the molar absorptivites for Co-DHBATSC and Ni-DHBATSC at their λmax were 445.20 and 274.95 L/mol cm3 respectively. In the absence of any surfactant these values of ε were 250.20 and 127.77 L/mol cm3 for Co(II) and Ni(II) complexes respectively. These results are summarized in Table (1-b). However, the use of a nonionic surfactant also enhances the stability of the two complexes. The complex in micellar medium become more soluble because of the forces between the negative head of the micelles and the positive charge of the metal ion causes a lower apparent formation constant between metal ions and ligands.[12]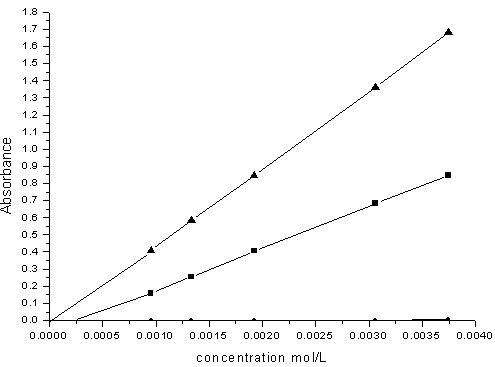 | Figure (5). Calibration curve of Co-DHBATSC (◄) at 525nm & Ni- DHHBATSC (■) complex at 480nm,C DHBATSC 2.5 ×10-3M , pH7 in Triton X-100(10%v/v) |
3.4. H-point Standard Addition Method
As indicated in Fig. (1), the appropriate wavelength pair for applying HPSAM is 463 and 500 nm. The analyte signal of Co(II) ions are linear with concentration, whereas, the interferent signal of Ni(II) remains constant by changing the analyte concentration at the selected wavelengths. To insure good accuracy, the Co(II) ion is considered as analyte and Ni(II) is the interferent, because the spectrum of Co-DHBATSC complex showed a large difference in absorbances at those wavelengths.
3.5. Reproducibility of HPSAM
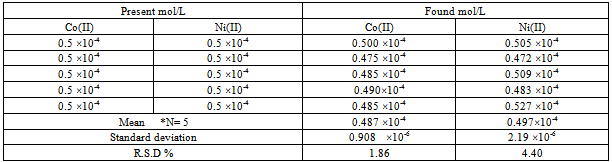
Simultaneous determination of cobalt and nickel under optimum conditions were performed. To examine the reproducibility of HPSAM, five replicate experiments of binary samples of Co(II) and Ni(II) were done, and Table (2) presents the results. The relative standard deviations (R.S.D) were 1.86 and 4.40 % for 5.00×10-5 M of each Co(II) and Ni(II) respectively. Table (1-a). Effect of surfactant on absorbances of cobalt and nickel complexes
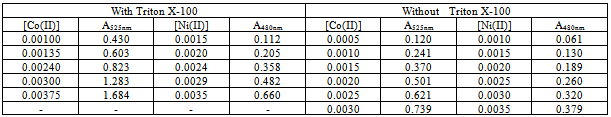 |
| |
|
Table (1-b). Statistical analysis of Co(II) and Ni(II) complexes
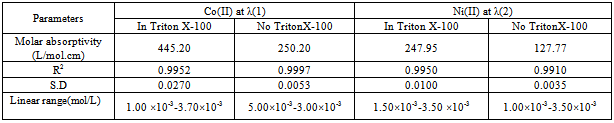 |
| |
|
Table (2). Results for five replicate for simultaneous determination of cobalt and nickel by HPSAM
 |
| |
|
3.6. Analysis of Cobalt-Nickel Binary Mixtures
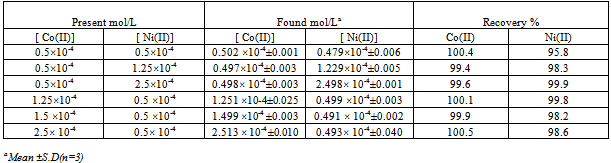
Several synthetic mixtures with different concentration ratios of Co(II) and Ni(II) were analyzed to test the accuracy of HPSAM for the simultaneous determination of both ions. The calibration curves at the selected wavelengths were plotted as shown in Fig. (6) and (7). The concentration of Co(II) (analyte) and Ni(II) (interferent), were determined by the ordinary values of CH and AH respectively. The results are given in Table (3).Table (3). Simultaneous determination of cobalt and nickel ions in binary mixtures by HPSAM (single determination)
 |
| |
|
Figs. 6 and 7 show the H-point standard addition plots for several synthetic test solutions with constant concentration of Co(II) and Ni(II) ions respectively. These Figures demonstrate the independent of CH on the Ni(II) concentration and the independent of AH values on Co(II) concentration.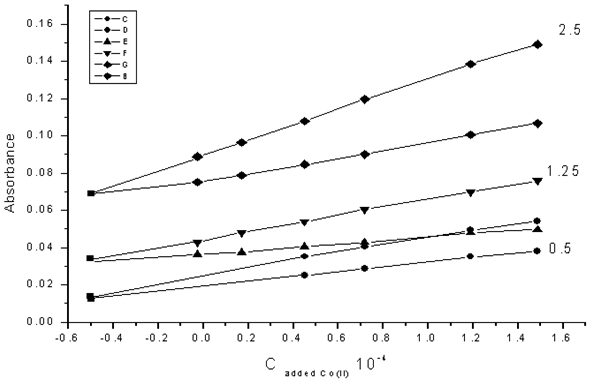 | Figure (6). H-point standard addition plot for the simultaneous determination of cobalt and nickel under optimized conditions with a constant concentration of cobalt (0.50 ×10-4 M) and nickel concentrations of: 0.5 × 10-4 M, 1.25 × 10-4 M and 2.5 × 10-4 M |
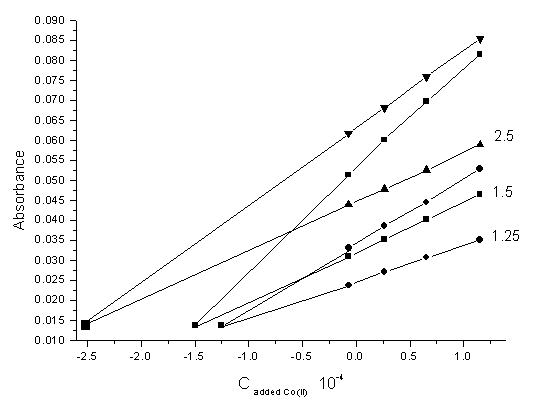 | Figure (7). H-point standard addition plot for the simultaneous determination of cobalt and nickel under optimized conditions with a constant concentration of nickel (0.50 × 10-4 M) and cobalt concentrations of: 1.25 × 10-4 M, 1.5 × 10-4M and 2.5 × 10-4 M |
3.7. Effect of Foreign Ions
The effect of the interference of some cations on the absorption signal of 1.0×10-4 M cobalt(II) and 1.0 × 10-4 M nickel(II) in a mixture were studied. The cations were considered to interfere when its presence produce a variation in the absorbance of the sample greater than 5%. Table (4) shows the interfering effect. Fe(III) and Pb(II) show positive effect on the signal, whereas Cu (II) and Cd(II) exhibit negative effect on the signal, therefore the presence of these cations must be removed in order to obtain accurate and precise results. Table (4). Effect of foreign cations on the determination of Co+2 and Ni+2 complexes
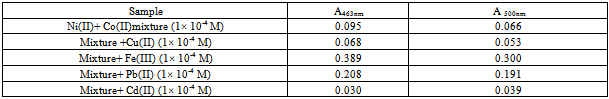 |
| |
|
4. Application of HPSAM
The proposed method was successfully applied to the determination of cobalt and nickel in spiked tap water, biscuits, and green tea samples. Also HPSAM was applied for analyzing cobalt in vitamin B12 tablets. The results were summarized in Table (5). Table (5). Determination of cobalt and nickel in different samples by HPSAM
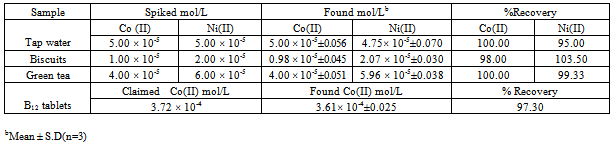 |
| |
|
The good agreement between the obtained results and the known added values indicate the applicability of HPSAM for simultaneous determination of nickel and cobalt in real samples.
5. Conclusions
In our best of knowledge this is the first simultaneous determination of cobalt and nickel by the H-point standard addition method, using new synthesized color reagent in micellar medium. The procedure showed enhancement in the sensitivity in micellar media. HPSAM provides satisfactory results in synthetic mixtures and spiked samples; thus the method can be applied to real samples. The developed method is simple, cheap, rapid and precise.
References
| [1] | A.P. Kumar, P.R. Reddy and V.K. Reddy, J. Korean Chem. Soc., 2007,51,4. |
| [2] | Nai-Laing Hu, Hong-Wen Gao, Biao Zhang and Guo-Qing Zhan, J.Chin.Chem.Soc. , 2005, 52, 1145-1152. |
| [3] | L.Pyeng-Huang, S. D. Kumar and S. Da Huang, J. Anal. At. Spectrom, 2001, 16,409-412. |
| [4] | M. A. Arain, F. H. Watto, M. H. S. Watto, A. B. Ghanghro, S. A. Tirmizi, J. Igbal and S. A. Arain, Arabia J. Chem., 2009, 2, 43-48. |
| [5] | V.Kaur, A.Kumar and N.Verma, J. Chin.Chem.Soc., 2007. 54, 715-722. |
| [6] | S.Puri, R.K.Dubey, B.K. Puri and M.F.Hussain, Analytical letters, 1998,31, 2,283-297. |
| [7] | H. Zhang, R. Wollast,V. Jean-Claude and G. J. Patriache, Analyst, 1989,114, 1597-1602. |
| [8] | E. N. Iliadou, S. T. Girousi, U. Dietze, M. Oho, A. N. Voulgaropoulos and C.G.Papadopoulos, Analyst, 1997, 122, 597-600. |
| [9] | S. B.Adeloju and A. Hadijchari, Analytical Sciences, 1999, 15, 95-100. |
| [10] | P.P. Kaur and U. Gupta, E.Journal of Chemistry,2009, 6,1, 106-112. |
| [11] | K.W.Tan, Y. Farina, C.H. Ng, M.J. Maah and S.W. Ng, Acta Cryst, 2008, 64, 1073. |
| [12] | H. Eskandari and Y. Kamali, Anal. Sci., 2004, 20, 1095-1098. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML






