-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2012; 2(6): 74-79
doi: 10.5923/j.aac.20120206.01
Spectrophotometric Determination of Ciprofloxacin Hydrochloride in Ophthalmic Solution
Edith Cristina Laignier Cazedey, Hérida Regina Nunes Salgado
School of Pharmaceutical Sciences, São Paulo State University, Araraquara, São Paulo, Brazil
Correspondence to: Edith Cristina Laignier Cazedey, School of Pharmaceutical Sciences, São Paulo State University, Araraquara, São Paulo, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The objective of this study was to develop simple and rapid method for the determination of ciprofloxacin hydrochloride in ophthalmic solution using UV spectrophotometry. The intrinsic absorbance of ciprofloxacin was measured directly at 275 nm. The calibration graph was linear over the range 2.0–7.0 µg mL-1 with relative standard deviations (RSD) values ranging from 1.55 to 2.47% (n=6). The method was validated through the parameters of linearity, accuracy, precision, limit of detection, limit of quantitation,specificity and robustnessaccording to ICH. The proposed method is enabled a quantitative determination of ciprofloxacin hydrochloride in ophthalmic solution.
Keywords: UV Spectrophotometry, Quinolones, Analytical Method, Ciprofloxacin Hydrochloride
Cite this paper: Edith Cristina Laignier Cazedey, Hérida Regina Nunes Salgado, "Spectrophotometric Determination of Ciprofloxacin Hydrochloride in Ophthalmic Solution", Advances in Analytical Chemistry, Vol. 2 No. 6, 2012, pp. 74-79. doi: 10.5923/j.aac.20120206.01.
Article Outline
1. Introduction
- Ciprofloxacin (1 – cyclopropyl – 6 – fluoro-1,4 – dihydro – 4 – oxo – 7-(1-piperazinyl) – 3-quinolone carboxylic acid) (Fig. 1) is a synthetic fluoroquinolone antimicrobial agent. It is a relatively new, second-generation fluoroquinolone antibiotic with an expanded spectrum of activity against Gram-positive and Gram-negative bacteria. This antimicrobial act through the inhibition of DNA-gyrase, an enzyme that is critical to bacterial chromosome replication. Ciprofloxacin, like other fluoroquinolones, contains a piperazine group at position 7 of the 4-quinolone nucleus, which results in activity against Pseudomonas aeruginosa. It is used in a wide range of infections of the urinary, respiratory and gastrointestinal tracts, as well as in skin structure and ocular infections[1].
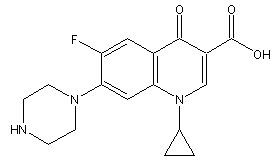 | Figure 1. Chemical structure of ciprofloxacin |
2. Material and Methodology
2.1. Reagents and Chemicals
- All reagents were analytical grade and high purity water was used in all experiments. A stock standard solution of ciprofloxacin hydrochloride (100.0 µg mL-1) was prepared using the standard from EMS Sigma Pharma Group (Brazil) and water. The solution was stored at 4℃. Working standard solutions were prepared from the stock solution by appropriate dilution with water.Ciprofloxacin hydrochloride ophthalmic solutions were obtained commercially. The ophthalmic solution were claimed to contain 3.5 mg mL-1 of drug and boric acid, sodium citrate, dissodiumedetate, benzalconium chloride and purity water as excipients.
2.2. Apparatus
- A UV/VIS spectrophotometer KAYAK XA, HP 8453 model was used with 1 cm optical path length quartz cells for all absorbance measurements.
2.3. Standard Preparations
- Aliquots of the stockstandard solution containing 100.0 µg mL-1 of ciprofloxacin hydrochloride were diluted to give the final concentrations of 2.0 to 7.0 µg mL-1. Water was used as blank. The absorbance was measured at 275 nm.
2.4. Sample Preparations
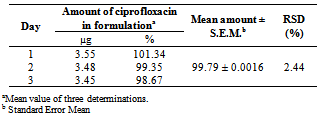
- Aliquots of ciprofloxacin hydrochloride ophthalmic solution were transferred volumetrically to give a final concentration of 3.5 µg mL-1. All determinations were conducted in six replicates.
3. Results and Discussion
3.1. Spectral Characteristics
- Several solvents were tested (water, methanol, hydrochloric acid 0.1 M and sodium hydroxide 0.1 M). The analytepresented maximum absorption at 275 nm in water (Fig. 2). This solvent was chosen because its excellent absorption, moreover itseconomic and low environment impacts.
3.2. Analytical Parameters
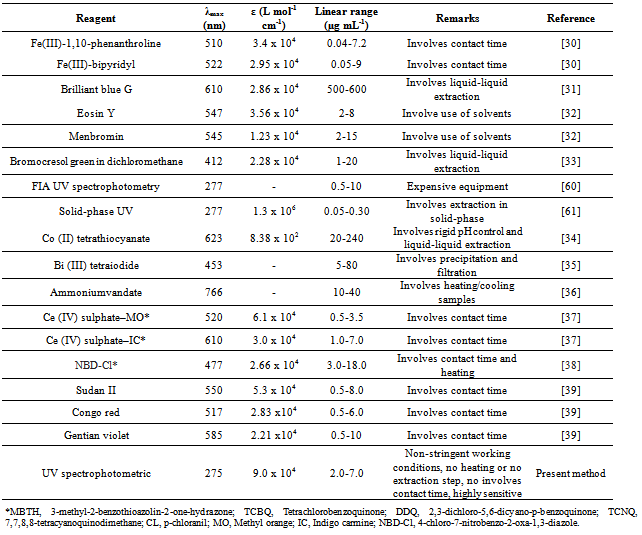
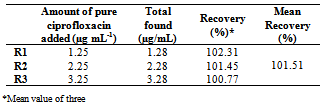
- The method showed linear regression in the concentration range of 2.0 to 7.0 µg mL-1(Fig.3). The precision of the proposed method was calculated by carrying out six independent assays of ciprofloxacin hydrochloride reference standard at concentration of 3.5 µg mL-1. The relative standard deviation (RSD) of six assays was calculated. The inter-day variation was similarly evaluated in three different days. The RSD was 2.30% and 3.04% for intraday and inter-day, respectively. The limit of detection (LOD) and limit of quantitation (LOQ) of ciprofloxacin hydrochloride by the proposed method were determined using analytical curves according to ICH [63], using the standard deviation of y-intercept of regression equation and the slope of the calibration curves.The proposed method was compared with others spectrophotometric methods found in the literature for the determination of ciprofloxacin (Table 1). As can be seen, the proposed method shows the highest sensitivity in terms of the apparent molar absorptivity (ε), so allowing analysis of lower concentration of ciprofloxacin.
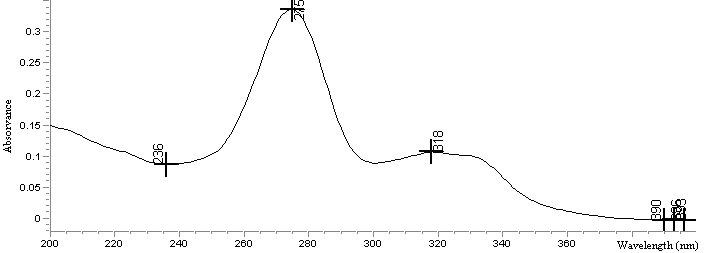 | Figure 2. Spectra absorption of ciprofloxacin hydrochloride in aqueous solution |
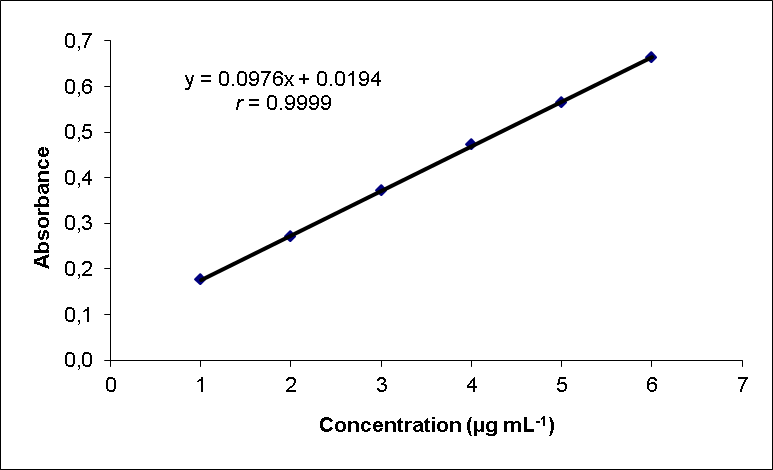 | Figure 3. Standard curve of ciprofloxacin hydrochloride |
|
|
|
4. Conclusions
- The proposed UV spectrophotometric method shows interesting features such as rapidity, simplicity, high sensitivity and low cost per analysis, involving neither sophisticated nor expensive instrumentation. The procedure is, therefore, suitable for the determination of ciprofloxacin hydrochloride in ophthalmic solution as a routine method with adequate accuracy and precision.
ACKNOWLEDGMENTS
- The authors thank the EMS Sigma Pharma Group (São Paulo, Brazil) for providing ciprofloxacin hydrochloride reference substance. This work was supported by PACD-FCFar-UNESP (Araraquara, Brazil), CNPq (Brasília, Brazil), FAPESP (São Paulo, Brazil) and CAPES (São Paulo, Brazil).
References
| [1] | Campoli Richards, D.M.; Monk, P.J.; Price, A.; Benfield, P.; Todd, P.; Ward, A.; Drugs 1988, 35, 373. |
| [2] | Husain, S.; Khalid, S.; Nagaraju, V.; NageswaraRao, R.; J. Chromatogr. A 1995, 705, 380. |
| [3] | Lacroix, P.M.; Curran, N.M.; Sears, R.W.; J. Pharm. Biomed. Anal. 1996, 14, 641. |
| [4] | Zotou, A.; Miltiadou, N.; J. Pharm. Biomed. Anal. 2002, 28, 559. |
| [5] | Kassab, N.M.; Singh, A.K.; Kedor-Hackmam, E.R.M.; Santoro, M.I.R.M.; Braz. J. Pharm. Sci. 2005, 41(4), 507. |
| [6] | Cazedey, E.C.L.; Perez, D.P.; Perez, J.P.; Salgado, H.R.N.; Chromatographia, 2009, 69, S241. |
| [7] | United States Pharmacopoeia (USP); 31th ed. United States Pharmacopoeia Convention: Rockville, 2008. |
| [8] | British Pharmacopoeia. London: Her Majesty's Stationery Office, 2003. |
| [9] | Farmacopéia Brasileira. 4.ed. São Paulo: Atheneu, 1988. |
| [10] | Mathur, S.C.; Lal, S.; Murugesan, N.; Rathore, Y.K.S.; Sethi, P.D.; Indian Drugs 1990, 27, 398. |
| [11] | Ramana Rao, G.; Avadhanulu, A.B.; Vasta, D.K.; IndianDrugs 1990, 27, 532. |
| [12] | Shanbag, S.G.; Thampi, P.P.; Thampi, C.S.; Indian Drugs 1990, 28, 279. |
| [13] | Bhowal, S.K.; Das, T.K.; Anal. Lett. 1991, 24(1), 25. |
| [14] | Bilgic, Z.; Tosunoglu, S.; Buyuktimkin, N.; Acta Pharm. Turc. 1991, 33, 19. |
| [15] | Sultan, S.M.; Suliman, F.E.O.; Analyst 1992, 117, 1523. |
| [16] | Tosunoglu, S.; Savci, N.; Acta Pharm. Turc. 1993, 35, 1. |
| [17] | Chowdary, K.P.R.; Rama Prasad, Y.V.; Indian Drugs 1994, 43, 277. |
| [18] | Sastry, C.S.P.; Rama Rao, K.;Prasad, D.S.; Talanta 1995, 42(3), 311. |
| [19] | El-Walily, A.F.; Belal, S.F.; Barry, R.S.; J. Pharm. Biomed. Anal. 1996, 14(5), 561. |
| [20] | Fratini, L. ; Schapoval, E.E.S. ; Int. J. Pharm. 1996, 127, 279.. |
| [21] | Suliman, F.E.O.; Sultan, S.M.; Talanta 1996, 43, 559. |
| [22] | Xuan, C.S.; Ren, S.C.; Song, J.L.; Wang, Z.Y.; YaowuFenxiZazhi 1996, 16, 164. |
| [23] | Bharat, P.V.; Rajani, G.; Vanita, S.; Indian Drugs 1997, 34, 497. |
| [24] | Abdel-Gawad, F.M.; Issa, Y.M.; Fahmy, H.M.; Hussein, H.M.; Mickrochim. Acta 1998, 130, 35. |
| [25] | Xuan, C.S.; Wang, Z.Y.; Song, J.L.; Anal. Lett. 1998, 31(7), 1185. |
| [26] | Avadhanulu, A.B.; Mohan, Y.R.R.; Srinivas, J.S.; Anjaneyulu, Y.; Indian Drugs 1999, 36, 296. |
| [27] | Amin, A.S.; Mikrochim. Acta 2000, 134, 89. |
| [28] | Rizk, M.; Belal, F.; Ibrahim, F.; Ahmed, S.M.; El-Enany, N.M.; Sci. Pharm. 2000, 68, 173. |
| [29] | Rizk, M.; Belal, F.; Ibrahim, F.; Ahmed, S.M.; Sheribah, Z.A.; J. AOAC Int. 2001, 84, 368. |
| [30] | Mostafa, S.; El-Sadek, M.; Alla, E.A.; J. Pharm. Biomed. Anal. 2002, 27, 133. |
| [31] | Nagaralli, B.S.; Seetharamappa, J.; Melwanki, M.B.; J. Pharm. Biomed. Anal. 2002, 29(5), 859. |
| [32] | Gowda, B.G.; Seetharamappa, J.; Anal. Sci. 2003, 19, 461. |
| [33] | El-Brashy, A.M.; Metwally, M.E.-S.; El-Sepai, F.A.; Il Farmaco 2004a, 59, 809. |
| [34] | El-Brashy, A.M.; Metwally, M.E.-S.; El-Sepai, F.A.; Bull. Korean Chem. Soc. 2004b, 25(3), 365. |
| [35] | El-Brashy, A.M.; Metwally, M.E.; El-Sepai, F.A.; J. Chin. Chem. Soc. 2005a, 52, 77. |
| [36] | El-Brashy, A.M.; Metwally, M.E.; El-Sepai, F.A.; J. Chin. Chem. Soc., 2005b, 52(2), 253. |
| [37] | Salem, H.; Am. J. Appl. Sci. 2005, 2, 719. |
| [38] | Basavaiah, K.; Nagegowda, P.; Somashekar, B.C.; Ramakrishna, V.; Sci. Asia 2006, 32, 403. |
| [39] | Abdel-Hay, M.H.; Hassan, E.M.; Gazy, A.A.; Belal, T.S.; J. Chin. Chem. Soc. 2008, 55(4), 818. |
| [40] | Amin, A.S.; Moustafa, M.E.; El-Dosoky, R.M.S.; Anal. Lett. 2008, 41, 837. |
| [41] | Ahmad, A.K.S.; Kawy, M.A.; Nebsen, M.; Anal. Lett. 1997, 30, 809. |
| [42] | El-Khateeb, S.Z.; Razek, S.A.A.; Amer, M.M.; J.Pharm. Biom. Anal. 1998, 17, 829. |
| [43] | Liu, J.; Han, B.; Chin. J. Antib. 1999, 24, 317. |
| [44] | Marona, H.R.N.; Schapoval, E.E.S.; J. Antimicrob. Chemother. 1999, 44(1), 136. |
| [45] | Hopkala, H.; Kowalczuk, D.; Acta Pol. Pharm. 2000a, 57(1), 3. |
| [46] | Hopkala, H.; Kowalczuk, D.; Pharmazie 2000b, 55(6), 432. |
| [47] | Kumar, K.G.; Chowdary, K.P.R.; Rao, G.D.; Indian J. Pharm. Sci. 2000, 37, 230. |
| [48] | Milovanovic, L.; Kapetanovic, V.; Popovic, G.; Dugumovic, Z.; Miljevic, D.; Aleksic, M.; Pharmazie, 2001, 56(2),150. |
| [49] | Altiokka, G.; Atkosar, Z.; Can, N.O.; J. Pharm. Biomed. Anal. 2002, 30, 881. |
| [50] | 50. Hopkala, H.; Kowalczuk, D.; J. AOAC Int. 2003, 86(2), 229. |
| [51] | Li, D.U.; Cao, X.M.; J. Shaanxi Normal University 2003, 1, 63. |
| [52] | Gomes, G.C.;Salgado, H.R.N.; Acta Farm. Bonaer. 2005, 24(2), 406. |
| [53] | Salgado, H.R.N.; Oliveira, C.L.C.G.; Pharmazie 2005, 60(4), 263. |
| [54] | Maheshawari, R.; Chaturvedi, S.; Jain, N.; Indian J. Pharm. Sci. 2006, 68, 195. |
| [55] | Sivasubramanian, L.; Muthukumaran, A.; Indian J. Pharm. Sci. 2006, 68(5), 672. |
| [56] | Hurtado, F.K.; Nogueira, D.R.; Bortolini, F.; Silva, L.M.; Zimmermann, E.; Souza, M.J.; Melo, J.; Rolim, C.M.B.; J. Liq. Chromatogr. Relat. Technol. 2007, 30(13), 1981. |
| [57] | Motwani, S.K.; Chopra, S.; Ahmad, F.J.; Khar, R.K.; Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 250. |
| [58] | Liu, R.L.; Xu, J.R.; Liu, Y.G.; Yao, Z.; YaowuFenxiZazhi 1994, 14, 45. |
| [59] | Zhang, L.T.; Sun, Z.X.; Chen, R.H.; Zhou, Z.X.; Zhou, Y.M.; Zhang, A.H.; Liu, W.N.; Whang, Z.H.; YaowuFenxiZazhi 1997, 17, 33. |
| [60] | Bungalowala, A.; Jain, D.K.; Trivedi, P.; Indian Drugs 1998, 35, 348. |
| [61] | Pascual-Reguera, M.I.; Parras, G.P.; Díaz, A.M.; J. Pharm. Biomed. Anal. 2004, 35, 689. |
| [62] | Pascual-Reguera, M.I.; Parras, G.P.; Díaz, A.M.; Microchem. J. 2004, 77, 79. |
| [63] | International Conference on Harmonization (ICH): Validation of Analytical Procedures: Text and Methodology, Geneva, IFPMA, 2005 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML