-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2012; 2(5): 67-73
doi: 10.5923/j.aac.20120205.04
Quatitative Methods for the Identification of Cefuroxime Sodium
Daniela Cristina de Macedo Vieir , Hérida Regina Nunes Salgado
Department of Drugs and Medicines, School of Pharmaceutical Sciences, UNESP – UnivEstadualPaulista, Araraquara, 14801-902, Brazil
Correspondence to: Daniela Cristina de Macedo Vieir , Department of Drugs and Medicines, School of Pharmaceutical Sciences, UNESP – UnivEstadualPaulista, Araraquara, 14801-902, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
From this study, methods for quantitative analysis were developed to determine cefuroxime sodium in the raw material and in pharmaceutical formulations. These tests included instrumental methods such as spectrophotometry in the ultraviolet, visible and infrared, high performance liquid chromatography and potentiometric determination by microbiological methods (agar diffusion and turbidimetric). The concentration range determined for each method varied greatly, which increases the usability of cefuroxime fori dentifying quality control. In spectrophotometric methods cefuroxime was determined from 5.0μg/ml to 2.0mg/mL.The microbiological methods have determined the drug concentration 30.0-120.0 μg/ml and HPLC ranged from 10.0to15.0μg/ml.The results obtained by analysis of the dosage form were compared to results obtained by analysis of the reference substance and showed significance. These methods proved to be reproducible and rapid determination of cefuroxime can be routinely used in quality control analysis.
Keywords: Quantitative Analysis, Quality Control, Cefuroxime
Cite this paper: Daniela Cristina de Macedo Vieir , Hérida Regina Nunes Salgado , "Quatitative Methods for the Identification of Cefuroxime Sodium", Advances in Analytical Chemistry, Vol. 2 No. 5, 2012, pp. 67-73. doi: 10.5923/j.aac.20120205.04.
Article Outline
1. Introdution
- Cefuroxime is a second generation cephalosporin first commercially available to be widely used in therapy.In the last decade the study of the cephalosporin molecule has been supported by the acquisition of knowledge of structure-activity relationships of potential sites for replacement of the parent molecule cephalosporin and allows the "construction" of molecules increasingly powerful[1].Since the first cephalosporin administered orally, cephalexin was licensed in 1968, progress has been made in the development of analogues more stable beta-lactamase with a broad spectrum antibacterial and pharmacokinetic properties and better metabolic: cefuroxime sodium follows this tendency.Cefuroxime {4 - (carbamoxylomymethyl) -8-[2-2-furyl-2-methoxyimino-acetyl]amino-7-oxo-2-thia-6-azabicyclo[4.2.0] oct-4-ene-5-carboxylic acid} (Figura 1) is a second generation cephalosporin injection is structurally similar to other third-generation cephalosporin such as cefotaxime and ceftazidime[2].It is highly effective and safe for the treatment of respiratory and urinary infections, but not active in vitro against Pseudomonas, Helicobacter (Campylobacter), methicillin resistant Staphylococcus aureus and S. epidermidis[3].
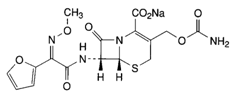 | Figure 1. Structure of cefuroxime sodium |
2. Materials and Methods
2.1. Collection and Sample Preparation
- We used reference substance (Batch G060123), kindly provided by Glaxosmithkline (Rio de Janeiro, Brazil). The commercial samples (powder for solution for injection) for cefuroxime sodium were purchased from Cellofarm Pharmaceuticals under the trade name Zencef®. Each vial contains cefuroxime sodium equivalent to 750 mg - Batch: 7400444. The reference substance presented declared purity of 97.4%, purified water used in the preparation of the solutions were obtained by the Milli-Q system (Millipore®, California) and all reagents and solvents used were of analytical grade.
2.2. Spectrophotometric Methods
- a) Ultraviolet spectrometry: ultraviolet spectrum was obtained in UV-VIS spectrophotometer Shimadzu UV-1240 using mini quartz cells of 1 cm optical path. The reading was performed at 280 nm. The calibration curve was determined in solutions prepared with water in concentrations ranging 5-14 μg/mL of sodium cefuroxime substance. Validation parameters were determined and after they became the determination of cefuroxime sodium in pharmaceutical form in triplicate.b) Spectrophotometry in the visible region: the spectrum in the visible region was obtained using UV-VIS spectrophotometer equipment Shimadzu UV mini-1240 and quartz cells of 1 cm optical path. Measurements were taken at 510 nm, and the calibration curve determined with solutions prepared in purified water at concentrations ranging from 100.0 to 300.0 μg/mL. Were added to each solution 1 ml of solution of Fe (III) and 0.01 M solution of 500 mL of 0.02 M o-phenanthroline. Validation parameters were determined and after they became the determination of cefuroxime sodium in pharmaceutical form in triplicate. c) Spectrophotometry in the infrared region: we used conventional spectrophotometer Shimadzu (Columbia, USA) with scan spectra that allows for analysis of electronic files. Equivalent weight were taken at 0.5, 0.7, 1.0, 1.5 and 2.0 mg of cefuroxime sodium reference substance (previously diluted in potassium bromide 1:10) and diluted in potassium bromide (Merck PA ) previously dried to constant weight and the pellets were prepared for reading with a total of 250 mg per tablet. The absorbance readings were performed in order to evaluate the intensity of peaks. Validation parameters were determined and after they became the determination of cefuroxime sodium in pharmaceutical form in triplicate.
2.3. Methods Microbiological
- a) Agar diffusion assay: the culture media, as well as purified water, were sterilized by autoclaving at 121°C for 15 minutes. Petri dishes were used with 20.0mm high and 100.0 mm diameter stainless steel and templates. These materials were sterilized in an oven at 200°C for 1 hour. Were chosen Micrococcus luteus (ATCC 9341) as standard microorganism at a concentration of 2.0%, means No. 1 and No. 2 Grove-Randall for base layer and surface respectively and water as diluent solution concentrations of the drug in 30.0, 60.0 and 120µg/ml. The standardization of the assay was performed using 3 x 3 technique described for other antimicrobial agents[16]. Validation parameters were determined and after they are made to determine the potency of cefuroxime sodium in pharmaceutical form in triplicate.b) Turbidimetric method: test tubes were used with 20.0 cm high and 4.0 cm in diameter were sterilized by autoclaving at 121°C for 15 minutes, and the purified water. For the incubation of microorganisms used to shaker incubator unit Marconi MA420® model for bacterial culture and greenhouse ECB Digital 1.2 (Odontobrás®). The readings were taken in a spectrophotometer BeckmanDU® 530 model. Micrococcus luteus were chosen (ATCC 9341) microorganism as standard at a concentration of 2.0% BHI broth and water as the diluent solution of the drug concentrations 30.0, 60.0 and 120 µg/mL. Validation parameters were determined and after they are made to determine the potency of cefuroxime sodium in pharmaceutical form in triplicate.
2.4. Chromatographic Methods
- a) High performance liquid chromatography: analyses were performed on liquid chromatograph Waters 1525 Binary HPLC with UV detection Tump/Vis (Milford, MA), manual injector Rheodyne 7725i Breeze and employed 054,275 Wat C18 column (Milford, MA) dimensions 150 mm x 4.6 mm and particle size of 5 μm and pre-column Waters. The mobile phase was prepared from a mixture of methanol (HPLC grade) and water (purified by reverse osmosis) in the ratio 70:30, v:v, filtered through a membrane of 0.45 μm in pore size and 47 mm diameter, under vacuum, and degassed for 15 minutes. After stabilization of the system were injected preset volumes of 20.0 µL of solution previously filtered through a membrane. The calibration curve was constructed with solutions of concentration between 10.0 to 15.0 µg/ml of cefuroxime sodium. Validation parameters were determined and after they became the determination of cefuroxime sodium in pharmaceutical form in triplicate.
3. Results and Discussion
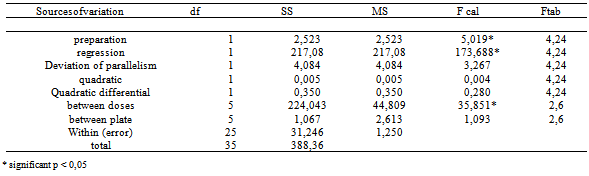
- Ultraviolet spectrophotometry: the ultraviolet absorption spectra are useful tools in the identification of organic compounds and elucidation of its structure, however detailed the relationship between molecular structure with absorption bands is quite extensive, but information relating to a compound of unknown structure can sometimes be obtained by direct comparison of its absorption spectrum with the model compounds of known structure[17]. The calibration curve was made by plotting absorbance values versus the concentration of reference substance and Beer's Law was valid in the range 5.0 to 14.0 µg/mL. The correlation coefficient (0.9996) and the equation of the line slope, y = 0.0412x - 0.0328 were calculated using the method of least squares. The molar absorptivity value was 1.15 x 104L mol-1cm-1. The repeatability of the method was determined by analysis of six replicates of the sample, each at concentration of 10 µg/mL. At this concentration, the relative standard deviation was 1.40%. The effectiveness of the method was evaluated by calculation of the F-test (variance ratio) and compared with the official method[18; 19]. Measurements of F-test values were obtained with 95% confidence (Table 1). The results show that the calculated F (2.81) does not exceed the theoretical value of 3.11 which is not significantly different from the official method[20].The accuracy was determined by the recovery test. Was obtained in this test recovery of 100.83% which is satisfactory and is a mandatory requirement for the validation of analytical methods. The accuracy of the method was obtained using six determinations of cefuroxime sodium injection, each preparation being carried out in triplicate and the measurements of different preparations. The robustness evaluated the reliability of the method proposed by changing the pH, which rose from 7.2 to 6.8 and by changing the value of that wavelength was changed of 280.0 nm and 283.0 nm. It can be seen that even with the change in these parameters the values found in three determinations of cefuroxime sodium in the product are in the level set that is 99-102% of the content. The average content of cefuroxime sodium in vials was 749.19 mg (99.89%) determined for six consecutive days and in triplicate, and each measurement originates from distinct solutions. The spectrophotometric method has low specificity for compounds containing β-lactam ring which absorb in the range of 250-270 nm[21]. However, the proposed method and the literature[22] show that this method has a good specificity and selectivity for the determination of sodium cefuroxime in the injectable pharmaceutical form.
|
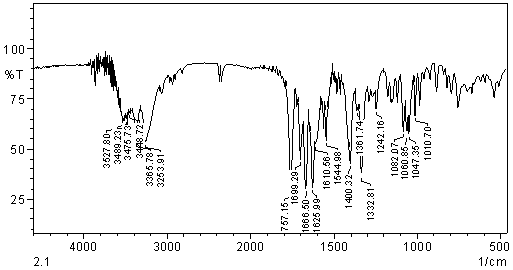 | Figure 2. IR spectrum in the region of cefuroxime sodium sample into KBr pellets |
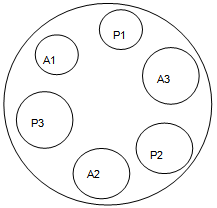 | Figure 3. 3 x 3 Design for cefuroxime bioassay, where: S: standard and T: test. S1 (30 μg/mL); S2 (60 μg/mL); S3 (120,0μg/mL); T1 (30 μg/mL); T2 (60 μg/mL) and T3 (120,0 μg/mL) |
|
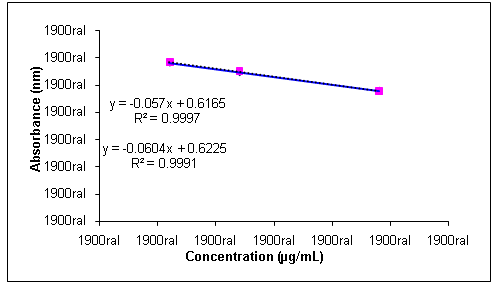 | Figure 4. Analytical curves for solutions of sodium cefuroxime reference substance and sample in concentrations of 30.0, 60.0 and 120.0 µg/mL, obtained by turbidimetric method |
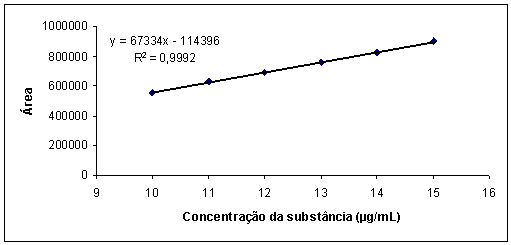 | Figure 5. Graphic representation of the analytical curve of the solution in concentrations of cefuroxime sodium 10-15 µg/mL by HPLC |
4. Conclusions
- The chemical and physicochemical properties of a drug are in many cases determinants in the process of analytical quality control, so that the chemical nature of the molecule can favorably or unfavorably interfere with the mechanisms of interaction with other substrates, making it more selective. Moreover, different methods, simple or complex are emerging every day in an attempt to become more viable and operational routine analysis of a substance. In our work we quantification of cefuroxime (widely sold in the Brazilian market) in raw material and in pharmaceutical preparations, showed to be reproducible to identify it and distinguish it, may be adopted in routine analysis of quality control industrial pharmaceuticals
ACKNOWLEDGEMENTS
- Authors thank GlaxoSmithkline Ltda for providing cefuroxime reference substance. This work was supported by PACD-FCFAr-UNESP-Brazil, FUNDUNESP-Brazil,FAPESP-Brazil and CNPq-Brazil.
References
| [1] | Neu HC. Beta-lactam antibiotics: structural relationships affecting in vitro activity and pharmacologic properties. Rev Infect Dis. 1986; 8: 237-259. |
| [2] | Amin AS, Ragab GH. Spectrophotometric determination of certain cephalosporins in pure form and in pharmaceutical formulations. SpectrochimActa. 2004; 60: 2831-2835. |
| [3] | Brunton LL,Lazo JS, Parker KL. Goodman & Gilman: As Bases Farmacológicas da Terapêutica, New York:McGraw-Hill Book Co.2006. |
| [4] | Adamis G,Papaioannou MG,Giamarellos-Bourboulis EJ, et al.Pharmacokinetic interactions of ceftazidime, imipenem and aztreonam with amikacin in healthy volunteers. Antimicrob. AgentsChemother2004;23: 144-149. |
| [5] | Altinoz S,Ozer D,Temizer A, et al.Determination of ceftriaxone in aqueous humour and serum samples by differential-pulse adsorptive stripping voltametry. Analyst. 1994; 119: 1575-1577. |
| [6] | AlyFA,Hefnawy MM,Belal, FA. Selective spectro-fluorimetric method for the determination of cephalosporins in biological fluids. Anal. Lett. 1996; 29: 1-10. |
| [7] | Dimitrovska A, Andonovski B, Stojanoski K. Spectrophotometric study of copper(II) ion complexes with cefaclor. Int. J. Pharm. 1996; 134: 213-221. |
| [8] | Yang JH, et al. Simultaneous determination of cephalexin and cephadroxil by using the coupling technique of synchronous fluorimetry and H-point standard additions method. Anal. Chim. Acta. 1996; 325: 195-200. |
| [9] | Reddy GVS, Reddy SJ. Estimation of cephalosporin antibiotics by differential pulse polarography. Talanta. 1997; 44: 627-631. |
| [10] | El-Mammly MY. Spectrophotometric determination of flucoxacillin in pharmaceutical preparations some nitrophenols as a complexing agent. Spectrochim. Acta. 2003; 59: 771-776. |
| [11] | Adamis G,et al. Pharmacokinetic interactions of ceftazidime, imipenem and aztreonam with amikacin in healthy volunteers. Antimicrob. Agents Chemother. 2004;23: 144-149. |
| [12] | Zivanovic L. et al. Investigation of chromatographic conditions for the separation of cefuroxime axetil and its geometric isomer.J. Chromatogr. B. 2004; 800: 175-179. |
| [13] | Tozo GCG, Salgado HRN. Determination of lomefloxacin in raw material and tablet preparations by liquid chromatography.J. AOAC Int. 2006; 89: 1305-1308. |
| [14] | Aly, HM, Amin AS. Utilization of ion exchanger and spectrophotometry for assaying amoxycillin and flucloxacillin in dosage form. Int. J. Pharm.2007; 338: 225–230. |
| [15] | Tozo GCG, Salgado HRN. Microbiological assay for cefoxitin sodium in dosage form. J. AOAC Int.2007; 90: 452-455. |
| [16] | FARMACOPEIA brasileira. 5.ed. São Paulo: Atheneu, 2010. 5.5.3.3.1, p. 264. |
| [17] | Ewing GW. Métodos instrumentais de análise química. São Paulo: Edgar Blücher, 2001. |
| [18] | THE UNITED STATES PHARMACOPEIA (USP 32). The National Formulary (NF 34). By authority of United States PharmacopeialConvention. Rockville, MD: United States Pharmacopoeial Convention, INC, 2011b. p. 683. |
| [19] | BRITISH Pharmacopoeia 2010. London: The Stationary Office, 2010. v. 2, p. 2475. |
| [20] | Rodrigues PC. Bioestatística. Rio de Janeiro: Universitária, 1986. p. 98-100. |
| [21] | Martinèz LG, Falcó PC, Cabeza AS. Comparison of several methods used for the determination of cephalosporins. Analysis of cephalexin in pharmaceutical samples. J. Pharm. Biomed. Anal. 2002; 29: 405-423. |
| [22] | Chaudhari, et al. Simultaneous UV spectrophotometric method for the estimation of cefuroxime axetil and probenecid from solid dosage forms. Indian J. Pharm. Sci. 2006; 68: 59-63. |
| [23] | Watson, DG. Pharmaceutical analysis: a textbook for pharmacy students and pharmaceuticals chemists. London: Churchill Livingstone, 1999. 337p. |
| [24] | Ohannesian; S. Handbook of Pharmaceutical Analysis. New York: Informa HealthCare, 2002. 585p. |
| [25] | Al-Momani, I.F. Spectrophotometric determination of selected cephalosporins in drug formulations using flow injection analysis. J. Pharm. Biomed. Anal. 2001; 25: 751-757. |
| [26] | Morgano MA, et al. Determinação de proteína em café cru por espectroscopia NIR e regressão PLS. Cienc. Tecnol. Alim, 2005; 25: 25-31. |
| [27] | Adams E, et al. Neomycin: microbiological assay or liquid chromatography? J. Pharm. Biomed. Anal. 1998; 17: 757-766. |
| [28] | Hewitt, W. Microbiological assay for pharmaceuticals analysis: a rational approach. New York: Academic Press, 2004. |
| [29] | Riley CM,Rosanske TW. MicroAnalytica. Anal. Chim. Acta. 1997; 338: 362. |
| [30] | BRASIL. Leis, decretos, etc. Resolução nº 899 de 29 de maio de 2003. Guia para validação de métodos analíticos e bioanalíticos. Diário Oficial da República Federativa do Brasil, DF, 02 jun. 2003. |
| [31] | Snyder LR, Kirkland JJ,Glajch JL. Practical HPLC method development. 2nd. ed. New York: John Wiley & Sons, 1997. v. 1, p. 292-316. |
| [32] | Watson, D.G. Pharmaceutical analysis: a textbook for pharmacy students and pharmaceuticals chemists. London: Churchill Livingstone, 1999. 337p. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML