-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2012; 2(5): 60-66
doi: 10.5923/j.aac.20120205.03
The Effect of 1-Methyl-3-butylimadazolium Tetrafluoroborate Ionic Liquid as a Mobile Phase Additive on the Retention Behavior of Nitroaromatic Explosives and Related Compounds
Tariq Ahmad , Samantha Smith , Bartlomiej Redlinski , Criag Utterback , Deonna Perkins , Stephanie Sharp , Ashley Heagy , Tarab Ahmad
Department of Chemistry, Western Illinois University, One Circle Dr., 324 Currens Hall, IL 61455, Macomb
Correspondence to: Tarab Ahmad , Department of Chemistry, Western Illinois University, One Circle Dr., 324 Currens Hall, IL 61455, Macomb.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this study, the use of the ionic liquid 1-butyl-3-methylimidazolium terafluoroborate (BMIM[BF4]) as an additive in the separation of mixtures containing nitroaromatic explosives and related compounds in reversed-phase high performance liquid chromatography is reported. The analytes used are: 1,3-DNB, 1,3,5-TNB, 2-A-4,6-DNT, 2,4-DNT, HMX, NB, RDX, TNT, 2NT, 3NT, 4 NT, tetryl, 2,6-DNT and 4A-2,6-DNT. The effects of the concentration of the ionic liquid on the retention, capacity factors and resolution of these analytes were studied. Mobile phases containing of 10% methanol with 0, 5, 10 and 20 mM BMIM[BF4], 20% methanol with 0, 5 and 20 mM BMIM[BF4] and 100% of 20 mM BMIM[BF4] were used for this study. All the mobile phases containing ionic liquids gave smaller capacity factors for the analytes and a run time that is smaller by 10-20 minutes compared to mobile phases containing methanol as a modifier. The number of compounds that have full or partial resolution is affected also by the concentration of the ionic liquid in the mobile phase. The elution order of the analytes was not affected by the addition of ionic liquids. These results show the potential application of ionic liquid BMIM[BF4] as a mobile phase additive to improve the resolution, decrease the analysis time and to reduce the organic solvent consumption in the HPLC analysis of the nitroaromatic explosives and related compounds.
Keywords: Ionic Liquids, Explosives, TNT, 1-methyl-3-butyl Imidazolium Tetrafluoroborate, HPLC
Cite this paper: Tariq Ahmad , Samantha Smith , Bartlomiej Redlinski , Criag Utterback , Deonna Perkins , Stephanie Sharp , Ashley Heagy , Tarab Ahmad , "The Effect of 1-Methyl-3-butylimadazolium Tetrafluoroborate Ionic Liquid as a Mobile Phase Additive on the Retention Behavior of Nitroaromatic Explosives and Related Compounds", Advances in Analytical Chemistry, Vol. 2 No. 5, 2012, pp. 60-66. doi: 10.5923/j.aac.20120205.03.
Article Outline
1. Introduction
- Significant amounts of the disposed explosives contaminate soil and ground water each year all over the world. This requires accurate monitoring and determination of the extent of contamination with these compounds. The identification of explosive residues is also significant to public and regulatory authorities in crime scene forensic investigation, environmental site remediation and homeland security. The most widely used explosives are trinitrotoluene (TNT), Octahydro-1,3,5,7 tetranitro-1,3,5,7 tetrazocine (HMX) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and its corresponding metabolites. These three compounds contaminate the soil and water during various military activities like manufacturing, testing and training, demilitarization and other operations. TNT and RDX are considered carcinogenic and are classified by EPA as emerging contaminants[1-2]. HPLCwith C18 reversed- phase and UV detection continues to be the main analytical technique for identification and verification of the presence of explosives. The USEPA specifies method 8330 and its revisions[3-7] for the trace analysis of nitroaromatic and associated nitroanialine residues in water, soil, or sediment matrixes. Analytes are able to be detected down to 2.5 ppb in water, soil or sediment. The EPA method documents co-elution of dinitrotoluene (DNT) and amino-dinitrotoluene (AmDNT) isomers on the primary C18 column. In order to identify and quantify all explosive compounds, it was necessary to rerun the samples under different chromatographic conditions and to use two chromatographic columns for the complete separation. Consequently, there was a concern about long term chromatographic performance and column-to column reproducibility issues. Several HPLC methods were developed for the analysis of these compounds[8-9]. However most of these methods use mobile phases that contain 40-50% methanol to comply with the conditions recommended in EPA method 8330. Therefore, these methods release large amounts of toxic wastes and increase the cost of the analysis without any significant reduction in the analysis time. Room temperature ionic liquids (RTILs) are salts with melting points lower or below ambient temperature which have unique properties. They have low to negligible vapour pressure. They are thermally stable, non-flammable, good solvents for a variety of inorganic and organic compounds, and recyclable. With all these properties, it is hoped that they can act as “green solvents” and they will replace volatile organic solvents in several chemical reactions[10-11]. The most commonly employed ionic liquids for the HPLC separations are the di-alkyl substituted imidazolium tetrafluoroborate salts. The advantages of using these ionic liquids in the HPLC analysis of several biochemical compounds and drugs are decreased band tailing, reduction of band broadening, and improvement of resolution[12-17]. These improvements are explained by the silanol blocking activity of the ionic liquids and have been associated with visible changes in retention and by their ability for intermolecular interactions of proton donor-acceptor types of coulombic types. In addition to the previous advantage of the ionic liquids in reversed-phase HPLC, the retention time of the analytes can be decreased or increased by changing the concentration of the ionic liquid in the mobile phase, the type of the anionic part of the ionic liquid and the length of the alkyl groups on the imidazolium ring. Therefore, ILs are very attractive tool in the hands of the separations scientists. The aim of the present study is to investigate the potential application the ionic liquid 1-methyl-3-butyl imidazolium tetrafluoroborate (BMIM[BF4]) as a mobile phase additives for the HPLC separation of different explosives and related compounds with the hope of developing a method in the future that would decrease the run time for their separation and that reduces the solvent consumption compared to the previous methods.
2. Experimental
2.1. Materials
- Explosives standards were obtained from Accu Standards (New Haven, CT). All solvents used (water and methanol) were of HPLC grade (Acros, New Jersey, USA). The ionic liquid 1-methyl, 3-butyl imidazolium tetrafluoroborate (BMIM[BF4]) was obtained from Sigma Aldrich. The concentration of the ionic liquid in the mobile phases was in the range 5-20 mM with or without methanol. Diluted standard solutions of the individual explosives (10-100) ppm were prepared daily by dissolving appropriate amounts of the stock solutions (1000 ppm) in methanol water 50:50 V/V mixtures. Mixtures of explosives (10-100 ppm) were prepared by mixing appropriate amounts of the individual solutions with the same solvent. For the sake of this study three types of mixtures were prepared, mixture 8 (mix 8) contains eight compounds 1,3-DNB, 1,3,5-TNB, 2-A-4,6-DNT, 2,4-DNT, HMX, NB, RDX and TNT, mix 6 contains 2NT, 3NT, 4 NT, tartly, 2,6 DNT, 4A-2,6 DNT and mix 14, and it contains all the 14 compounds
2.2. Apparatus
- Shimadzu liquid chromatograph, HPLC-model 20A, equipped with auto sampler (SIL 20A / 20 AC) & online degasser (DGU-20 A3 / DGU 20 A5) was used. The detector was UV-VIS detector (SPD-20A / SPD-20AV). The column used was Grace smart C18 (150mm, 4.6mm, 5μm). It was purchased from Grace Davison Discovery Sciences (Deerfield, IL, USA). The column was conditioned with the mobile phase for 1-5 hours depending on the type of mobile phase used. The solvent was composed of either aqueous ionic liquid solution 10 mM without methanol or with 30-50% methanol at a flow rate of 1.0 ml/min. Typical injection volumes were 10μL. Duplicate injections were made. The detection of the analytes was set at appropriate wavelength (254 nm). The dead volume of the column was measured by injecting thiourea. All the experiments were done at room temperature at 23C. The following equation were used for the capacity factor calculations k = (t-t0)/t0, where t stands for the retention time of the individual peak and t0 is the elution of the unretained sample (thiourea).
3. Results and Discussion
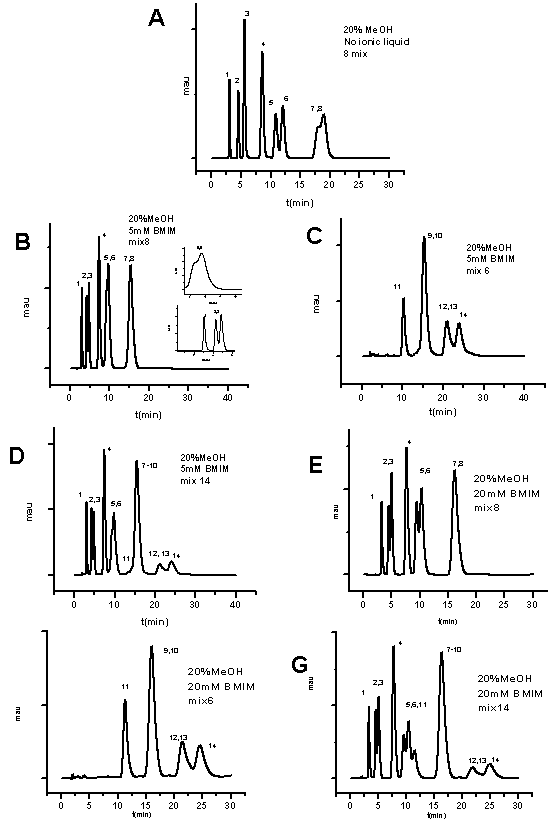
- Before investigating the effect of the BMIM[BF4] ionic liquid on the separation of the mixture of nitroaromatic explosives and related compounds, mobile phases containing different percentages of methanol 10-50% were used to separate the mixture of explosives that contains 6, 8 and 14 compounds. None of the mixtures were completely separated with these mobile phases. However, the mobile phase containing 10% was able to give complete baseline resolute of 6 compounds of mix 8(1, 3-DNB, 1, 3, 5-TNB, HMX, NB, RDX and TNT) and partial resolution of the last two eluting compounds (2-A-4, 6-DNT, 2, 4-DNT). For mix 6; the mixture containing the 6 compounds; 2NT, 3NT, 4 NT, tetryl 2, 6-DNT, 4A-2, 6-DNT, and only 4 peaks appeared in the chromatogram in which two compounds (tetryl and 2NT) have completely resolution, while the four other compounds eluted in two other peaks. For mix 14 in which the mixture contains all 14 compounds ((1,3-DNB, 1,3,5-TNB, 2-A-4,6-DNT, 2,4-DNT, HMX, NB, RDX, TNT, 2NT, 3NT, 4 NT, tetryl, 2,6-DNT and 4A-2,6-DNT) only 9 peaks appeared in the chromatogram in which the HMX, 1,3, 5 TNB, 1, 3 DNB, NB, 2NT are completely resolved, while the TNT and tetryl elute in one peak and the four other compounds 2, 6-DNT, 4-A-2,6DNT, 2, 4-DNT and 2-A, 4, 6-DNT elute in one peak The run time for eluting all 14 compounds together using the mobile phase containing 10% methanol was 65 minutes. Figure 1A-C shows the chromatograms for the three mixtures using mobile phases of aqueous solution of 10% methanol with no ionic liquid added. To study the effect of 1-butyl, 3-methylimidazolium tetraflouroborate ionic liquid on the separation of the mixtures, 6, 8 and 14, a mobile phase containing 5 mM BMIM[BF4] and 10% methanol was used. The chromatograms obtained for three mixtures are shown in Figure 1D-F. Mix 8 shows 7 peaks with complete separation of the main compounds RDX, HMX, TNT and the other compounds like 1,3-DNB, NB and 1,3,5 TNB. However, the two compounds 4 DNT and 2-A, 4, 6-DNT elute at the same time. For mix 6 and 14, the same number of peaks was obtained as with the mobile phase containing only 10% methanol without the ionic liquid. It is apparent form these chromatograms also that the run time for mix 8 was reduced by approximately 25 minutes, while it is reduced by 10 minutes for mix 6 and 14 compared to the experiments in which there was only 10% methanol in the mobile phase with no ionic liquid added. It is observed that the elution order of the compounds didn’t change compared with the elution order with 10% methanol.
4. Conclusions
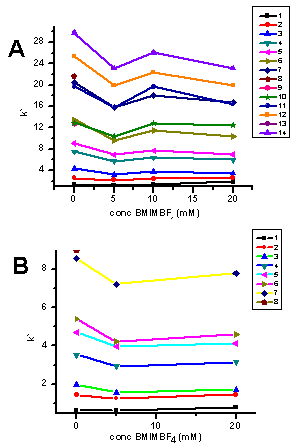
- It was demonstrated that the ionic liquid BMIM[BF4] is useful as a mobile-phase additive in RP chromatography of nitroaromatic explosives and related compounds. A very important feature of this additive is that it decreases the retention of these compounds without the need of increasing the amount of the organic modifier in the mobile phase. The capacity factor of the studied compounds is also affected by the concentration of the ionic liquid in the mobile phase. The mobile phase with 10 mM BMIM[BF4] gave the highest capacity factor for all the analytes. This study shows also that the elution order of the analytes using the ionic liquid as an additive is the same as the elution order with methanol as a modifier The resolution of the compounds can be changed by changing the concentration of the ionic liquid in the mobile phase or by changing both the ionic liquid and the methanol concentration. Future studies will be focused on using other chromatographic columns and other ionic liquids and using temperatures other than room temperature which is used for this study.
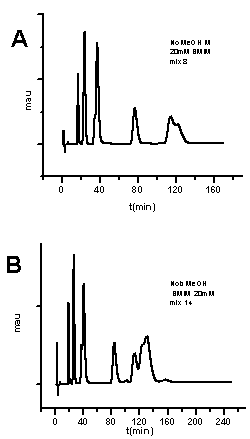 | Figure 4. Chromatograms of mixtures of 8, and 14 explosives using mobile phase containing 20 mM BMIM[BF4] and no methanol ;same experimental conditions as in Figure1 |
ACKNOWLEDGMENTS
- The authors of this manuscript would like to acknowledge the College of Arts and Sciences at Western Illinois University for their continuous and generous support for this research through the undergraduate research and travel funds.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML