-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2012; 2(5): 53-59
doi: 10.5923/j.aac.20120205.02
Application of Advanced Radio Analytical Technique in Characterization of Anion Exchange Resins Duolite A-368 and Duolite A-171
Pravin U. Singare
Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai, 400 058, India
Correspondence to: Pravin U. Singare , Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai, 400 058, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present study deals with the application of radio analytical technique for characterization of two anion exchange resins Duolite A-368 and Duolite A-171 by using 131I and 82 Br as a tracer isotopes. The characterization is made on the basis of kinetics of iodide and bromide ion-isotopic exchange reactions studied under different experimental conditions like temperature and concentration of exchanging medium. It is observed that both the resins the bromide and iodide ion-isotopic reaction rates in min-1 increases with concentration of exchanging medium and decreases with rise in temperature. In addition to exchange reaction rates, other parameters like distribution coefficient, amount of ion exchanged in mmol and percentage of ions exchanged are also calculated. The results indicate existence of strong positive co-relationship between amount of ions exchanged and concentration of ionic solution, while a strong negative co-relationship exist between amounts of ions exchanged and temperature of exchanging medium. The reaction rate and distribution coefficient values, amount and percentage of ions exchanged calculated for Duolite A-171 resins are higher than that obtained for Duolite A-368 resins. The overall results indicate superior performance of Duolite A-171 resins than Duolite A-368 resins under identical operational parameters.
Keywords: Duolite A-171, Duolite A-368, 131I, 82Br, Ion-Isotopic Exchange, Reaction Kinetics, Distribution Coefficient, Tracer Isotopes, Radio Analytical Technique, Tracer Technique
Cite this paper: Pravin U. Singare , "Application of Advanced Radio Analytical Technique in Characterization of Anion Exchange Resins Duolite A-368 and Duolite A-171", Advances in Analytical Chemistry, Vol. 2 No. 5, 2012, pp. 53-59. doi: 10.5923/j.aac.20120205.02.
Article Outline
1. Introduction
- Industrial applications of radioisotopes constitute a major part of the use of radioisotopes, the world over. The economic benefits that may be derived from the use of radioisotope technology are great, a fact that is recognized by the governments of developing countries. The availability of a wide spectrum of radioisotopes from the reactors and cyclotrons led to the quest for their beneficial applications in diverse fields such as medicine, industry and agriculture. The choice of the radioisotope and the physical and chemical property of the radiotracer to be used will depend on the nature of the study. In radiotracer study, a short lived radioisotope in a physico-chemical form similar to that of the process material is used to trace the material under study. The tracer concentration recorded at various locations is analyzed to draw information about the dynamic behavior of the system under study. The efficiency of several devices in a clarifiers, aeration tank) is investigated by means ofradiotracers[1]. Radiotracer methodology is described extensively in the literature[2-4]. Applications of radiotracers in chemical research cover the studies of reaction mechanism, kinetics, exchange processes and analytical applications such as radiometric titrations, solubility product estimation, isotope dilution analysis and autoradiography.Considering the above wide use of radioactive isotopes in various industrial and technical applications, in the present investigation, they are applied to assess the performance of industrial grade anion exchange resins Duolite A-368 and Duolite A-171 under different operational parameters like temperature and ionic concentrations. It is expected that the tracer technique used here can also be used forcharacterization of other organic ion exchange resins which are synthesized for their specific technical applications[5-7]. The present technique can also be extended further to standardize the operational parameters so as to bring about the most efficient performance of those resins in their specific industrial applications.
2. Experimental
2.1. Conditioning of Ion Exchange Resins
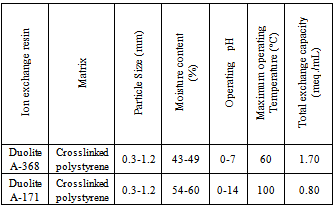
- Ion exchange resin Duolite A-368 is a weak base anion exchange resin in OH- form having –N+R2 functional group and Duolite A-171 is a strong basic anion exchange resin in Cl- form having –N+R3 functional group (supplied by Auchtel Product Ltd., Mumbai, India). Details regarding the properties of the resins used are given in Table 1. These resins were converted separately in to iodide / bromide form by treatment with 10 % KI / KBr solution in a conditioning column which is adjusted at the flow rate as 1 mL / min. The resins were then washed with double distilled water, until the washings were free from iodide/bromide ions as tested by AgNO3 solution. These resins in bromide and iodide form were then dried separately over P2O5 in desiccators at room temperature.
|
2.2. Radioactive Tracer Isotopes
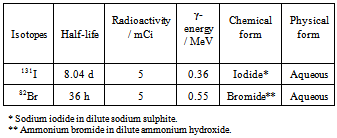
- The radioisotope 131I and 82Br used in the present experimental work was obtained from Board of Radiation and Isotope Technology (BRIT), Mumbai. Details regarding the isotopes used in the present experimental work are given in Table 2.
|
2.3. Study on Kinetics of Iodide Ion-Isotopic Exchange Reaction
- In a stoppered bottle 250 mL (V) of 0.001 M iodide ion solution was labeled with diluted 131I radioactive solution using a micro syringe, such that 1.0 mL of labeled solution has a radioactivity of around 15,000 cpm (counts per minute) when measured with γ -ray spectrometer having NaI (Tl) scintillation detector. Since only about 50–100 μL of the radioactive iodide ion solution was required for labeling the solution, its concentration will remain unchanged, which was further confirmed by potentiometer titration against AgNO3 solution. The above labeled solution of known initial activity (Ai) was kept in a thermostat adjusted to 30.0 °C. The swelled and conditioned dry ion exchange resins in iodide form weighing exactly 1.000 g (m) were transferred quickly into this labeled solution which was vigorously stirred by using mechanical stirrer and the activity in cpm of 1.0 mL of solution was measured. The solution was transferred back to the same bottle containing labeled solution after measuring activity. The iodide ion-isotopic exchange reaction can be represented as:
 | (1) |
2.4. Study on Kinetics of Bromide Ion-Isotopic Exchange Reaction
- The experiment was also performed to study the kinetics of bromide ion- isotopic exchange reaction by equilibrating 1.000 g of ion exchange resin in bromide form with labeled bromide ion solution in the same concentration and temperature range as above. The labeling of bromide ion solution was done by using 82Br as a radioactive tracer isotope for which the same procedure as explained above was followed. The bromide ion-isotopic exchange reaction can be represented as:
 | (2) |
3. Results and Discussion
3.1. Comparative Study of Ion-Isotopic Exchange Reactions
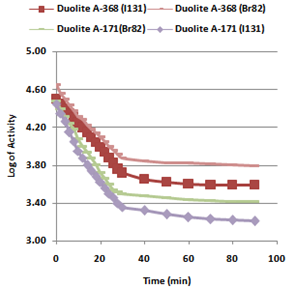
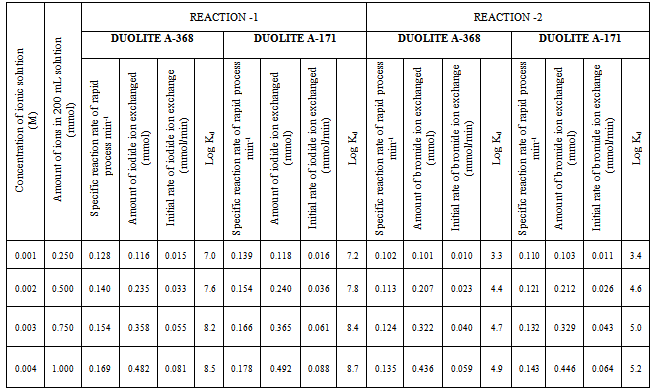
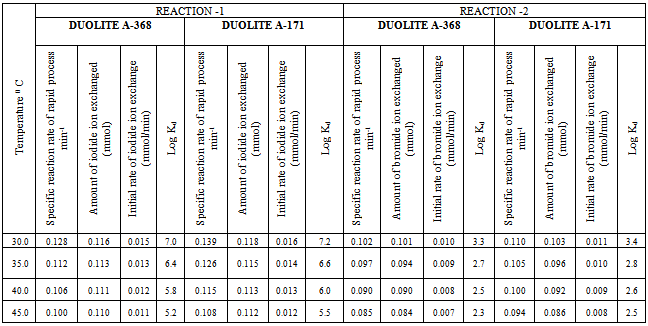
- In the present investigation it was observed that due to the rapid ion-isotopic exchange reaction taking place, the activity of solution decreases rapidly initially, then due to the slow exchange the activity of the solution decreases slowly and finally remains nearly constant. Preliminary studies show that the above exchange reactions are of first order[24, 25]. Therefore logarithm of activity when plotted against time gives a composite curve in which the activity initially decreases sharply and thereafter very slowly giving nearly straight line (Figure 1), evidently rapid and slow ion-isotopic exchange reactions were occurring simultaneously[9-23]. Now the straight line was extrapolated back to zero time. The extrapolated portion represents the contribution of slow process to the total activity which now includes rapid process also. The activity due to slow process was subtracted from the total activity at various time intervals. The difference gives the activity due to rapid process only. From the activity exchanged due to rapid process at various time intervals, the specific reaction rates (k) of rapid ion-isotopic exchange reaction were calculated. The amount of iodide / bromide ions exchanged (mmol) on the resin were obtained from the initial and final activity of solution and the amount of exchangeable ions in 250 mL of solution. From the amount of ions exchanged on the resin (mmol) and the specific reaction rates (min-1), the initial rate of ion exchanged (mmol/min) was calculated.Because of larger solvated size of bromide ions (310 pm) as compared to that of iodide ions (300 pm), it was observed that the exchange of bromide ions occurs at the slower rate than that of iodide ions[26]. Hence under identical experimental conditions, the values of specific reaction rate (min-1), amount of ion exchanged (mmol) and initial rate of ion exchange (mmol/min) are calculated to be lower for bromide ion-isotopic exchange reaction than that for iodide ion-isotopic exchange reaction as summarized in Tables 3 and 4. For both bromide and iodide ion-isotopic exchange reactions, under identical experimental conditions, the values of specific reaction rate increases with increase in concentration of ionic solution from 0.001M to 0.004M (Table 3). However, with rise in temperature from 30.0℃ to 45.0℃, the specific reaction rate was observed to decrease (Table 4). From the results, it appears that iodide ions exchange at the faster rate as compared to that of bromide ions which was related to the extent of solvation (Tables 3 and 4).
|
|
 | (3) |
3.2. Comparative Study of Anion Exchange Resins
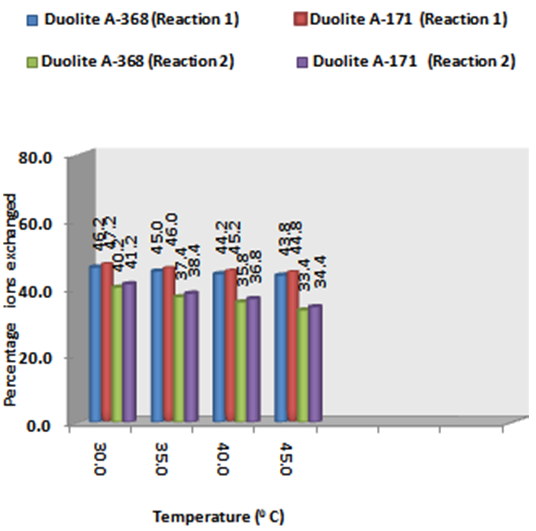
- From the Table 3, it is observed that for iodide ion-isotopic exchange reaction by using Duolite A-171 resin, the values of specific reaction rate (min-1), amount of iodide ion exchanged (mmol), initial rate of iodide ion exchange (mmol/min) and log Kd were 0.139, 0.118, 0.016 and 7.2 respectively, which was higher than 0.128, 0.116, 0.015 and 7.0 respectively as that obtained by using Duolite A-368 resins under identical experimental conditions of 30.0℃, 1.000 g of ion exchange resins and 0.001 M labeled iodide ion solution. The identical trend was observed for the two resins during bromide ion-isotopic exchange reaction. From Table 3, it is observed that using Duolite A-171 resins, at a constant temperature of 30.0℃, as theconcentration of labeled iodide ion solution increases 0.001 M to 0.004 M, the percentage of iodide ions exchanged increases from 47.2 % to 49.2 %. While using Duolite A-368 resins under identical experimental conditions the percentage of iodide ions exchanged increases from 46.2 % to 48.2 %. Similarly in case of bromide ion-isotopic exchange reaction, the percentage of bromide ions exchanged increases from 41.2 %to 44.6 % using Duolite A-171 resin, while for Duolite A-368 resin it increases from 40.2 % to 43.6 %. The effect of ionic concentration on percentage of ions exchanged is graphically represented in Figure 2.
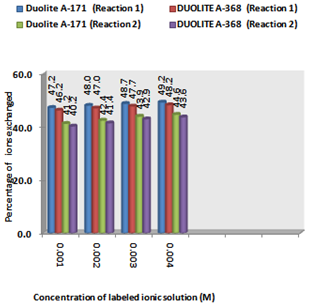 | Figure 2. Variation in Percentage Ions Exchanged with Concentration of Labeled Ionic Solution Amount of ion exchange resin = 1.000 g, Volume of labeled ionic solution = 250 mL, Temperature = 30.0℃ |
3.3. Statistical Correlations
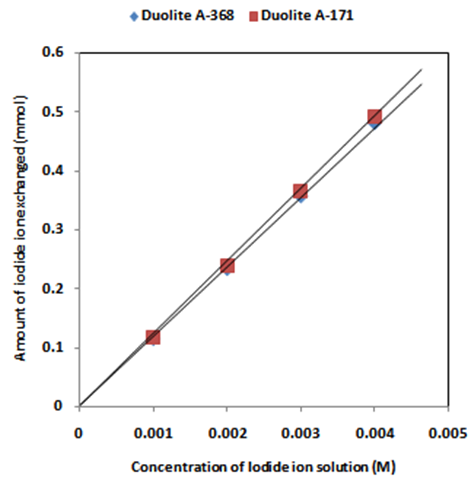
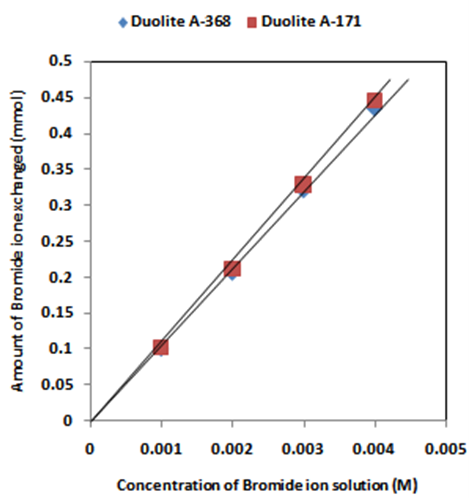
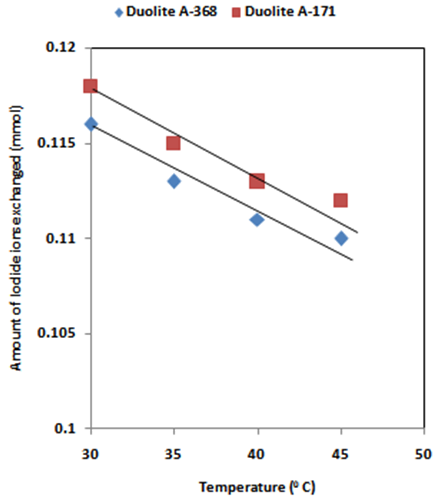
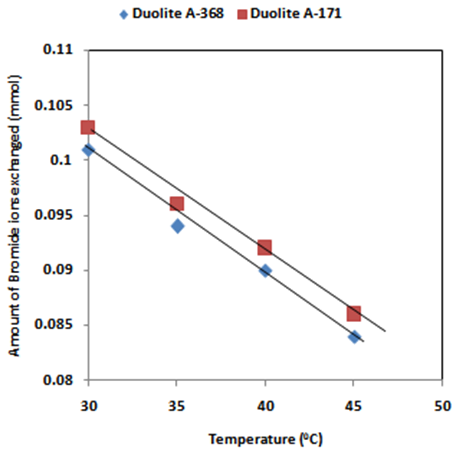
- The results of present investigation show a strong positive linear co-relationship between amount of ions exchanged and concentration of ionic solution (Figures 4, 5). In case of iodide ion-isotopic exchange reaction, the values of correlation coefficient (r) was calculated as 1.0000 for both the resins, while for bromide ion-isotopic exchange reaction, the values of r was calculated as 0.9999 and 0.9998 for Duolite A-171 and Duolite A-368 resins respectively. There also exist a strong negative co-relationship between amount of ions exchanged and temperature of exchanging medium (Figures 6, 7). In case of iodide ion-isotopic exchange reactions the values of r calculated for Duolite A-171 and Duolite A-368 resins was -0.9759. Similarly in case of bromide ion-isotopic exchange reactions the r values calculated was -0.9951 for both the resins.
4. Conclusions
- The experimental work carried out in the present investigation will help to standardize the operational process parameters so as to improve the performance of selected ion exchange resins. The radioactive tracer technique used here can also be applied for characterization of different nuclear as well as non-nuclear grade ion exchange resins.
ACKNOWLEDGEMENTS
- The author is thankful to Professor Dr. R.S. Lokhande for his valuable help and support in carrying out theexperimental work in Radiochemistry Laboratory of Department of Chemistry, University of Mumbai, Vidyanagari, Mumbai -58.The author is extremely thankful to SAP Productions for developing and maintaining the manuscript template.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML