-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2012; 2(4): 41-45
doi: 10.5923/j.aac.20120204.04
Development of a Predictive Model for Removal of Organic Matter from Leachate Landfill by Catalytic Oxidation Using Response Surface Methodology
Milton Rosero-Moreano 1, Cristina Nerín 2
1Grupo de Investigación en Cromatografía y Técnicas Afines GICTA, Departamento de Química, Universidad de Caldas. Calle 65 Nº 26-10 Sede Principal A.A: 275, Manizales-Caldas-Colombia
2Universidad de Zaragoza, I3A, Escuela de Ingeniería y Arquitectura EINA Campus Ebro Departamento de Química Analítica C/ Maria de Luna 3 50018 Zaragoza-Spain
Correspondence to: Milton Rosero-Moreano , Grupo de Investigación en Cromatografía y Técnicas Afines GICTA, Departamento de Química, Universidad de Caldas. Calle 65 Nº 26-10 Sede Principal A.A: 275, Manizales-Caldas-Colombia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work the heterogeneous Fenton catalytic experiments were arranged in a CCF experimental design and analyzed by response surface methodology, for every peroxide concentration. These response surfaces were the representation of a quadratic predictive model developed for improving of biochemical oxygen demand at five days/chemical oxygen demand (BDO5/COD) ratio, trough the optimization parameters of reaction. For this proposal 18 catalytic tests were carried out evaluating three main factors: peroxide concentration, catalyst loading and the peroxide addition rate. The results in confronting with the experimental data showed that from 60 minutes the catalytic wet peroxide oxidation (CWPO) reaction was stationated in focusing the chemical COD removal, inclusively in any opportunities these values were decreased maybe because new compounds had been formed and we supposed that these new compounds were with biodegradable character because this behavior was perfectly answered by the prediction model, hence with this new and enhancent ratio there are possibilities to get in to the biological process again for the refine the effluent and comply with the actual regulation. The best conditions predicted for the model are at 4.68M peroxide concentration, catalyst loading charge (CL) (0.5 – 0.6%) and peroxide addition rate (PAR) (7.5 – 10 mL/h) to improve its biodegradability to 0.30, thus this effluent might come back to the begin of plant for refining the treatment.
Keywords: Leachate of Landfill, CWPO, RSM
Article Outline
1. Introduction
- Landfill is nowadays the main way of disposal for the enormous charge of municipal solid wastes generated all around the world[1]. However, rain and liquid percolation throughout them provokes the side production of significant volumes of a strongly contaminant liquid, the so-called leachate of landfill. In general terms, it is a dark liquid of offensive odor, high loading of organic compounds and complex chemical composition, which usually includes not negligible charge of bio-reluctant compounds[2]. Catalytic wet peroxide oxidation CWPO is a green technology that meets the characteristics required to perform efficiently in the treatment of such highly contaminant streams. In CWPO the hydrogen peroxide is the source of the powerful hydroxyl radicals, whose generation can be activated by using inexpensive solid catalysts like Al/Fe-pillared clays, and the process can be efficiently carried out at very mild conditions of ambient temperature and pressure. The employment of a solid catalyst enables both, efficient immobilization of the active metal that avoids its recovery from the effluent as one extra step, as well as the catalyst reuse along several reaction catalytic cycles[3]. One of the tools that have broughtsignificant benefits when optimizing analytical processes in which several variables simultaneously affect the analysis, is the experimental design based on factorial design[4] and response surface methodology[5]The aim of this work was to develop a response surface methodology (RSM) to find a model able to predict the optimum parameters of reaction improving either, COD removal, BOD5/COD ratio of the output stream or both, employing for that purpose a central composite face (CCF) quadratic experimental design tool.
2. Experimental
2.1. Leachate Characteristics
- The leachate employed in the catalytic runs was perfectly described in Galeano et al[3] and all data used for this development are from the joint work at the University of Nariño [6]
2.2. Experimental Design
- The catalytic tests were carried out in two batches: one batch at 2.34 mol/L of the peroxide concentration (PC) and another one at 4.68 mol/L H2O2. In Table 1 it is shown the arrangement of the 18 catalytic tests carried out by duplicate using a central composite face (CCF) quadratic experimental design. The other two factors were tested at three levels each one: catalyst loading (CL) of 0.5, 1.0 and 2.0 (gNaBVAlFe3/100 mLleachate) and the peroxide addition rate (PAR) of 7.5, 15 and 30 (mL/h).
3. Results and Discussion
3.1. Design of Experiments
- Since all the trends till now found for every factor at a time have shown to strongly depend on the chosen conditions for the rest of parameters, their simultaneous observation was tried to get better insight of the experimental system. The central composite face design CCF constituted by a full or fractional factorial design and center points placed in the faces, is one class of response surface methodologies often used for fitting of second-order models in the design of experiments[7]. The CCF was used in this work, considering the minimum and maximum levels for H2O2 concentration (2.34 – 4.68 mol/L), catalyst loading (0.5 – 2.0 wt./V %), and rate of peroxide addition (7.5 – 30 mL/h) and two responses: COD removal and BI (BOD5/COD ratio). Assuming a second-order polynomial model, at least 18 replicated runs (36 experiments) must be carried out to solve the matrix, for which statistical software MODDE v6.0 was used. Each run was replicated to check the reproducibility and to evaluate the experimental error of the results obtained in the design of experiments (DOE). The 36 experiments are put together in the 18 replicated runs listed in Table 1. A random order was employed to perform the experiments in order to minimize systematic errors. As above mentioned, the targeted responses to be maximized were both, the COD removal and the BI value.
|
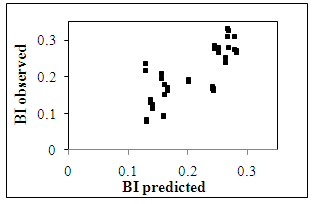 | Figure 1. Experimental and calculated results provided by the RSM model for output biodegradability index of leachate at 4 h of reaction |
3.2. Modeled Response for Biodegradability Index BI
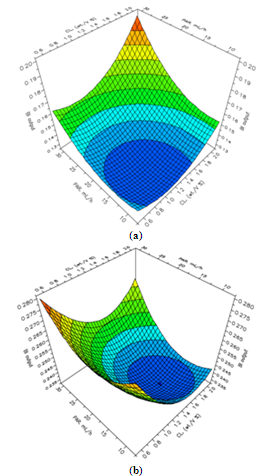 | Figure 2. Response surface plots for biodegradability index predicted at different peroxide concentrations. (a)[H2O2] = 2.34 mol/L; (b)[H2O2] = 4.68 mol/L |
3.3. Overall Performance
- The overall performance of the heterogeneous CWPO reaction must be carefully analyzed taking into account not only the modeled predicted BI response but also the experimental results obtained for the COD removal[3]. It is evident that high catalyst loadings together with low peroxide addition rates and peroxide dosages seem to promote a better use of the oxidizing agent towards COD removal, whose direct consequence drives to lower costs of operation. Furthermore, as said in last paragraph the better predicted reaction parameters for BI enhancement are at first apparently the opposite of those needed to achieve the highest COD removal. However, in Figure 2 (b) can be seen that BI response surface at high PC exhibits another maximum, though a bit less pronounced, for high CL and high PAR which can also be seen at low PC (see Figure 2 (a)). Run 18 in Table 1 meets such a pair of reaction parameters at high peroxide concentration and dosage, while shows a reasonable high COD removal close to 40 %. It indicates that modeled BI offers several possible sets of conditions to maximize its response, including some where moderate to high COD removals can also be achieved at the same time. It can be briefly summarized as follows. Although the commented lower intrinsic oxidizing power displayed by not-activated molecular hydrogen peroxide compared to hydroxyl radicals leads to increased biodegradability in the output leachate, it is not necessarily accompanied by a significant COD removal. Apparently, when the reaction is driven preferentially following such a pathway, there is an important BOD5 increment but scarce COD depletion, which anyway conduces to higher biodegradability in the output stream (see runs 11 and 12 at Table 1). In the other hand, as high catalyst loadings are employed, in fact if we speak about lower H2O2/Fe(s) mole ratios (Table 1) it could result more accurate, the role played by the catalytically-generated radical species becomes fairly more important. Under this scenario COD removal results clearly favored on BI enhancement (see runs 4 and 16 at Table 1) probably because the powerful radicals conduce to deeper oxidation and higher degree of mineralization, though generating lower fraction of by-products featuring enhanced biodegradability.Nevertheless, it must be stressed that such behavior strongly depends also on peroxide concentration and dosage.
4. Conclusions
- CCF experimental design was used to arrange the catalytic experiments, and a response surface methodology was applied to data, where an acceptable predicting model was obtained only for BI response while poor fitting prevented to find out another one for COD removal response.
ACKNOWLEDGEMENTS
- M. Rosero-Moreano gratefully thanks to chemists Melba Tutalcha and Jazmin Figueroa for their precious work. The authors thanks also to Gobierno de Aragón (Spain), Grupo GUIA, Grupo consolidado de Investigación T-10.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML