-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2012; 2(4): 37-40
doi: 10.5923/j.aac.20120204.03
Flame Atomic Absorption Spectrometry Based on Self-absorption in the Flame and Using the Flame as a Light Emission Source
Anastas Dimitrov Dakashev 1, Stancho Valkanov Pavlov 2, Krasimira Angelova Stancheva 1
1Department of Inorganic and Analytical Chemistry, Prof. Dr. Assen ZlatarovUniversity, 8010 Bourgas, Bulgaria
2Department of Mathematics and Physics, Prof. Dr. Assen Zlatarov University, 8010 Bourgas, Bulgaria
Correspondence to: Anastas Dimitrov Dakashev , Department of Inorganic and Analytical Chemistry, Prof. Dr. Assen ZlatarovUniversity, 8010 Bourgas, Bulgaria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A method of flame atomic absorption analysis has been developed which does not need a light source, such as hollow-cathode lamp, which to produce the radiation absorbed by the analyte atoms. Light emission from the analyzed atoms in the flame can serve as a light source because this emission has the same wave length as the resonance absorption line of the unexcited analyte atoms. At certain conditions, in the flame can take place an absorption process known as self-absorption. In the work self-absorption occurring in the flame is used to determine absorbance. Absorbance is calculated from the flame emission intensity signal of the analyte atoms. A computer program is specially created to calculate absorbance, to draw the calibration curve and to compute the analyte concentration. For safety purposes ethyl alcohol is used as a flame fuel and is applied as an alcohol vapor / air flame. Because this flame has low temperature, only lithium, potassium, sodium, calcium, barium is able to be analyzed.
Keywords: Flame Atomic Absorption Analysis Base on Self-absorption, Flame As a Light Emission Source for Atomic Absorption, Determination of Absorbance by Flame Emission Data
Article Outline
1. Introduction
- In atomic absorption spectrometry the hollow-cathode lamp is a spectral line light source commonly used to produce the radiation absorbed by the analyte atoms. When the element to be analyzed is a non-metal, or when a hollow-cathode lamp produces a weak signal, an electrode-less discharge lamp is employed. A recently developed hollow-cathode lamp with boosted-discharge has improved analytical characteristics such as greater intensity, calibration graphs with less curvature, increased sensitivity in comparison to a conventional hollow- cathode lamp[1]. Also, a diode laser can be used as the light source for atomic absorption analysis[2]. Another current approach to performing atomic absorption analysis involves use of a continuum light source like a xenon arc lamp combined with a high resolution monochromator[3]. In the early development of atomic absorption spectroscopy, flame emission was used as a light source. This light source was prepared by aspirating a solution, containing a high concentration of the analyte, in a second flame. This method was later applied by Clifton Calloway Jr and Bradley Jones[4]. They claimed the light source was inexpensive and that setup changes to accommodate different analytes were quick and simple.In the present work, one flame both atomizes the analyte and provides the light emission source.
2. Principle of the Method
- In the flame, analyte atoms are excited and emit light. According to Kirchoff’s law, the emitted light has the wave length of the resonance absorption line of unexcited atoms. As emitted light passes from some part in the interior of the flame to the outside, it is absorbed by unexcited analyte atoms. This is well known as a self-absorption process. In the present work self-absorption is used to determine absorbance. In the particular case where light emitted by the exited analyte atoms in the flame serves as a light source, absorbance A is defined by the equation: A = log (Iat / If ), where Iat is the light emission intensity of the exited atoms in the flame, and If is the initial emission intensity reduced by self-absorption, or emission intensity of the light that leaves the flame. The value of If is measured in the conventional way, while Iat value has to be found in order for the absorbance to be determined. In the present work Iat is calculated. A computer program is specifically designed to make necessary calculations, as well as to draw the calibration curve, and determine the analyte concentration[5].
3. Experimental
3.1. Apparatus
- An atomic absorption spectrometer Unicam SP 90 Series 2, designed also for emission measurements, is used when acetylene / air flame is applied. When ethyl alcohol is used as a flame fuel, the nebulizer and the mixing chamber of the spectrometer are replaced by a nebulizer and a mixing chamber, taken from an AAS-1N spectrometer, and adapted to the Unicam spectrometer. Using a vaporizer, the fuel is introduced in the spectrometer as an alcohol vapor. A variety of burner heads are used as follows: 1. A Meker burner head for propane / air gas mixture, mentioned in the paper as MBH 120.7, consisting of 91 openings, each of 1.2 mm in diameter, situated in a circle, 25 mm in diameter, with a total opening section area of 120.7 mm2. 2. A Meker burner head for acetylene / air gas mixture, MBH 45.5, with 58 openings, each of 1.0 mm in diameter, situated in a circle, 15 mm in diameter, with a total opening section area of 45.5 mm 2. 3. A Meker burner head for acetylene / air, MBH 14.7, with 13 openings, each of 1.2 mm in diameter, situated in a square, 8×8 mm, with a total opening section area of 14.7 mm 2. 4. An acetylene / air three-slot burner head 3SBH 120, with dimensions of each slot: 50×0.8 mm, with a total opening section area of 120 mm 2. 5. An acetylene / air single slot burner head, slot dimensions: 100×0.8 mm. Use is also made of a burner, named 2MBH 120.7, composed of two Meker burner heads, MBH 120.7, arranged near each other in a line coinciding with the optical line of the spectrometer. This burner gives a flame with great dimensions.
3.2. Reagents
- All of the reagents used in the work are of analytical grade. Denatured alcohol is applied as a flame fuel.
3.3. Solutions
- Solutions are made up in double distilled water. The stock standard solutions are prepared directly by weighing the recommended solid reagents, dissolving and diluting to the corresponding volumes with double distilled water. The working standard solutions are prepared by suitable dilution of the stock solutions with double distilled water.
4. Results and Discussion
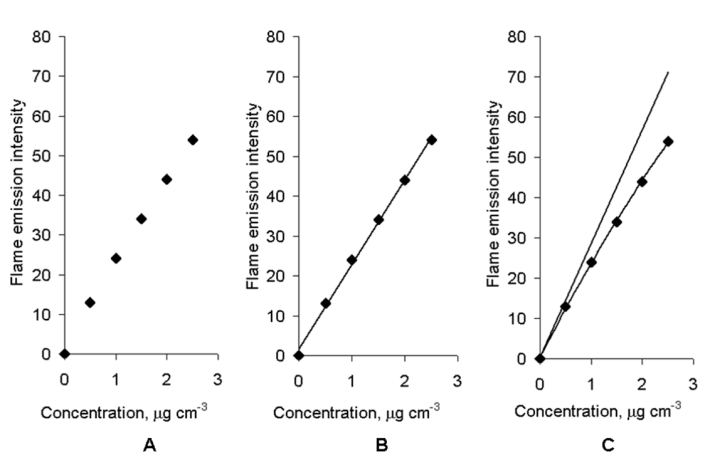
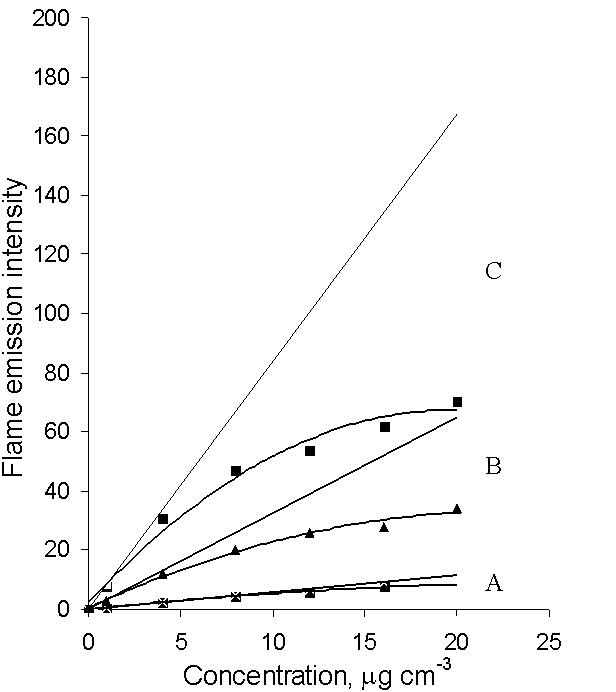
- The only difference between the flame atomic absorption process, occurring in ordinary flame atomic absorption analysis and the self-absorption process by the method under consideration, is the location of the light emission source. For the adsorption process, it is outside the flame but for self-adsorption process is inside it. Therefore, self-absorption must obey to the same laws as absorption. When self-adsorption in the flame occurs, intensity of the light that leaves the flame If is a nonlinear function of concentration C, according to Beer’s law: If = Iat 10 -aC, where a is absorptivity. When self-absorption in the flame does not take place, flame light emission intensity If is a linear function of analyte concentration (the basis of Flame emission spectrometry). That means the appearance of If function is a criterion for evaluating the self-adsorption process. Experimental data of flame emission intensity for different analyte concentrations are given in Fig.1 A.
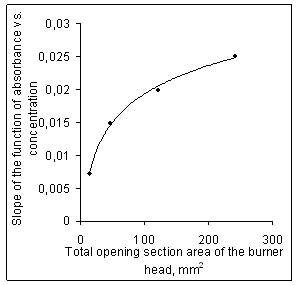 | Figure 3. Sensitivity of flame atomic absorption determination as a function of total opening section area of the burner head |
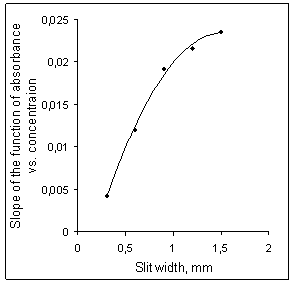 | Figure 4. Sensitivity of flame atomic absorption determination as a function of slit width of the spectrometer |
References
| [1] | Colin A. Watson, Use of Improved Design and Boosted-discharge Hollow-cathode Lamps, Royal Society of Chemistry Publishing, Journal of Analytical Atomic Spectrometry, vol.5, no.3, pp.404-414, 1988. |
| [2] | David Butcher, Lasers as Light Sources for Analytical Atomic Spectrometry, Taylor and Francis Ltd, Applied Spectroscopy, Reviews, vol. 42, no.6, pp. 543-562, 2007. |
| [3] | H. Becker-Ross, S. Florek, U. Heitmann, M.D. Huang, M. Okruss, B. Radziuk, Continuum Source Atomic Absorption Spectrometry and Detector Technology: A Historical Perspective, Elsevier, Spectrochimica Acta, Part B, Review, vol.61, pp. 1015–1030, 2006. |
| [4] | Clifton P Calloway Jr, Bradley T Jones, Atomic Absorption Spectrometry With a Flame Emission Source, Pergamon, Spectrochimica Acta, Part B, Review, vol. 49B, nos 12-14, pp. 1707-1715, 1994. |
| [5] | Anastas D. Dakashev, A Method for Performing Atomic Absorption Analysis Using the Atomizer as a Light Emission Source, Bulgarian Patent No 110535, 27.11.2009. |
| [6] | Anastas D.Dakashev, Maria M. Nedelcheva, Krasimira A. Stancheva, Flame Emission Spectrometric Analysis Using Ethil Alchohol as a Flame Fuel, University Publishing, Annual Assen Zlatarov University, vol. 37, no.1, pp.15-18, 2008. |
| [7] | Matthieu Chausseau, Emmanuelle Poussel, J.M. Mermet, Self-absorption effects in radially and axially viewed inductively coupled plasma-atomic emission spectrometry – the key role of the operating conditions, Springer, Fresenius’ Journal of Analytical Chemistry, vol.370, no. 4, pp. 341-347, 2001. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML