-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2011; 1(1): 1-7
doi: 10.5923/j.aac.20110101.01
Prodigious Azocalix[4] Pyrrole Super-molecule: Effective Reagent for Liquid-liquid Extraction, Preconcentration and Transport of Cu(II) Ions
Hiren C. Mandalia , Vinod K. Jain
Chemistry Department, School of Sciences, Gujarat University, Ahmedabad, Gujarat, 380009, India
Correspondence to: Vinod K. Jain , Chemistry Department, School of Sciences, Gujarat University, Ahmedabad, Gujarat, 380009, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A novel series of eight prodigious calix[4]pyrrole super-molecules bearing azo-moiety at the meso position of the macrocycle have demonstrated the ability for liquid-liquid extraction, preconcentration and transport of Cu(II) ions across a liquid membrane. Various significant extraction parameters such as effect of pH, effect of solvent and effect of reagent concentration were investigated which showed high affinity and selectivity towards Cu(II) in the presence of large quantities of associated metal ions. The wavelengths of maximum extraction (λ max) and molar absorptivity (ε) have also been determined. The stoichiometry of complex was evaluated by slope ratio method. The system obeys Beer’s law over the range 0.5–10.0 μg mL-1. Liquid membrane transport studies of Cu(II) were carried out from source to the receiving phase under controlled conditions and a mechanism of transport is proposed. To check the validity of the proposed method, Cu(II) was analyzed in natural and ground water samples of Ahmedabad city, Gujarat, India.
Keywords: Calix[4] Pyrrole, Azo-dyes, Copper, Liquid-liquid Extraction, Transport Study
Cite this paper: Hiren C. Mandalia , Vinod K. Jain , "Prodigious Azocalix[4] Pyrrole Super-molecule: Effective Reagent for Liquid-liquid Extraction, Preconcentration and Transport of Cu(II) Ions", Advances in Analytical Chemistry, Vol. 1 No. 1, 2011, pp. 1-7. doi: 10.5923/j.aac.20110101.01.
Article Outline
1. Introduction
- The design and synthesis of a variety of host molecules is a challenging topic in the field of molecular recognition chemistry because of the promising new functions of these novel compounds or the fantastic features attainable by forming supramolecular complexes from the host and guest molecules[1]. Synthetic chromo-ionophores that give rise to specific color changes on selective complexation with cations have attracted considerable attention as efficient spectrophotometric analytic reagents for the detection of particular species as well as the design of supramolecular devices having recognition and optical sensing functions[2-3]. Generally, the developments of most chromogenic reagents based on the supramolecular calixarenes have been achieved by attaching a chromogenic moiety/chelating moiety. There have been few reports wherein macrocycles such as calixarene[4], calixresorcinarene[5], thiacalixarene[6] and crown-ether[7] are used as components for the preparation of azo dyes but the research in the area of calix[4]pyrrole based dyes[8] is still in infancy. Important positive and negative roles of trace heavy metal ions in human health are known[9]. Lot of studies[10] has been performed for the determination of trace metal ions in various media including some body tissues and fluids, natural waters etc. Also the investigation of trace heavy metal contents in food samples including honey, vinegar, lemon juice, sour cream, yogurt, buttermilk, chocolate, cocoa, honey, molasses and other food samples are an important part of analytical chemistry[11].Copper has received considerable attention owing to its uses in metallurgy and chemical industries. Copper is a gastrointestinal tract irritant, but it is generally not harmful to humans at low (μg mL-1) concentrations. In surface water, however, copper can be toxic to aquatic plants[12] and some fishes at concentrations of less than 1.0 μg mL-1. Thus, copper tends to be much more of an environmental hazard than a human hazard. Most environmental and biological samples generally contain copper at ng level. Many industrial waste water streams (such as those used in metal works, semiconductor, and copper industries, mining etc.) contain heavy metals which are of great environmental concern and must be removed prior to water discharge or water recycling[13]. Therefore, from the viewpoints of pollution, environmental chemistry, geochemistry, marine biology and analytical control in industrial, food, agricultural, pharmaceutical and clinical areas, it is necessary to establish a rapid, simple, sensitive and accurate procedure for the selective concentration of copper prior to its determination. Numerous techniques[14] for the separation and concentration of metal ions including evaporation of solvents, electro-deposition, surface adsorption, precipitation, ion exchange, ion exchange impregnated materials, immobilized reagents, electro-osmosis and flotation have been reported. Some of these techniques may be tedious, having limited concentration factors, lengthy and rigid conditions for the separation of solid adsorbents[15]. For the determination of copper, various instrumental methods[16,17] including UV Vis spectrophotometry, FAAS, ET-AAS, ICP-AES, ICP-MS, spectrofluorimetry, anodic stripping voltammetry and ion chromatography have been used. ET-AAS, ICP-MS, and anodic stripping voltammetry can be directly applied to the determination of copper at the ng mL-1 level. However, some of these methods are time-consuming, require complicated and expensive instruments, have complex operational conditions, are not free from various types of interference, and have high maintenance costs. Spectrophotometry still represents an attractive technique for the determination of metal ions in aqueous media because of its simplicity, being inexpensive and ready availability[18]. So, the development of a simple, sensitive and reliable method for the determination of copper is worthwhile. Although a lot of chromogenic reagents[19] for detection of transition metals have been reported, none of the reagents was prepared by attaching a chromogenic moiety such as azo (–N=N-) functional group at the meso-position of a macrocyclic compound, such as calix[4] pyrrole. It is even more interesting and significant to develop a new type of calix[4]pyrrole based chromogenic reagent for detection of Cu(II). Since we synthesized a series of novel eight azocalix[4] pyrrole dyes[20] and carried out very preliminary complexation studies with Cu(II), we thought it worth to extend our investigations to liquid liquid extraction, preconcentration and transport of Cu(II) across a liquid membrane in a non polar solvent. The extracted copper complexes have been determined simultaneously by spectrophotometry/flame atomic absorption spectrometry (FAAS)/inductively coupl- ed plasma emission spectrometry (ICP-AES). Various parameters for the extraction have been studied. The validity of the proposed method is tested by analyzing this Cu(II) in natural and ground water samples of Ahmedabad city, Gujarat, India.
2. Experimental Section
2.1. Instruments and Measurements
- UV-Vis absorption studies were carried out on a JASCO 570 UV/VIS/NIR spectrophotometer using 10mm quartz cells. All pH measurements were performed using an Elico digital pH meter, model L1 614, equipped with a combined pH electrode. A Chemito, flame atomic absorption spectrophotometer (FAAS), model 201, equipped with air-acetylene burner was used for the analysis under conditions recommended by the manufacturer. The analytical wavelength for Cu(II) was 324.8 nm. Inductively coupled plasma atomic emission spectrophotometer (ICP-AES), plasma scan model 710 was used for the determination of trace metal content, only in those cases where concentration of metal ion was too low to be determined by spectrophotometry and FAAS.
2.2. Chemicals and Reagents
- All the chemicals used were of analytical grade from E. Merck or BDH. All aqueous solutions were prepared with quartz distilled deionized water, which was further purified by a Millipore Milli-Q water purification system (Millipack 20, Pack name: Simpak 1, Synergy) throughout the entire study. The pH was adjusted with the following buffer solutions[21]: PO4-3/HPO4-2 buffer for pH 2.0 and 3.0; CH3COO-1 /CH3COOH buffer for pH 4.0 and 6.0; HPO4-2/H2PO4-1 buffers for pH 7.0 and 7.5. Standard copper stock solution (1000 μg mL-1) was prepared by dissolving 0.3805 g of copper nitrate [Cu(NO3)2.3H2O] in 100 mL water and was standardized spectrophotometrically[22]. Working solution was subsequently prepared by appropriate dilution of the stock solution.The eight tetra functionalized azocalix[4]pyrrole dyes (Figure 1) were synthesized and characterized as per earlier reported method[20] and their stock solutions (0.1%) were prepared in iso-amyl alcohol. Working solutions were subsequently prepared by appropriate dilution of the stock solutions.
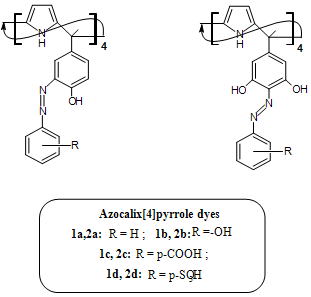 | Figure 1. Tetra functionalized azocalix[4]pyrrole dyes (1a-d, 2a-d) |
2.3. Sample Collection
- The water samples from Sabarmati River were isokinetically collected in clean polyethylene bottles from locations near a thermal power station, Ahmedabad. The ground water samples were collected from the university area and Vatva industrial zone of Ahmedabad city, Gujarat, India.
2.4. General Procedure for the Liquid-Liquid Extraction of Cu(II)
- An aliquot of sample solution containing 0.5-10 µg of Cu(II) was transferred into a 25 mL separatory funnel and desired 5 pH was adjusted with the appropriate buffer solutions in a total volume of aqueous phase of 10 mL. The mixture was shaken with 6 ml of 0.015 % reagents (azocalix[4]pyrrole dyes) in iso-amyl alcohol. The metal-reagent complex was extracted into the organic phase. To ensure complete recovery, the extraction was repeated with 2 mL of reagent solution; the organic extract was separated, dried over anhydrous sodium sulphate and transferred into a 10 mL volumetric flask. The combined extracts and washings were diluted to the mark (10 mL) with iso-amyl alcohol. The absorbance of the organic phase was measured at 540 nm.The concentration of the metal ion extracted into the organic phase [Cu+2](org) as complex was estimated by [Cu+2](org) = [Cu+2](aq, int) - [Cu+2](aq), where [Cu+2+](aq, int) is the initial concentration of the metal ion in the aqueous phase. The percent extraction (%E), was calculated by Eq. (1)
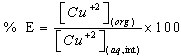 | (1) |
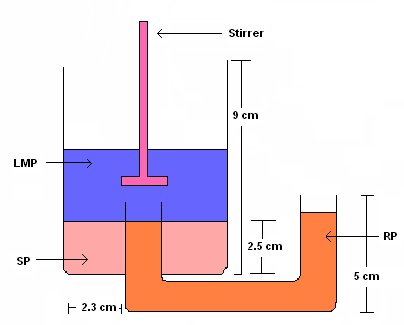 | Figure 2. Apparatus for Transport Studiesa |
2.5. Liquid Membrane Transport Studies
- Transport of Cu(II) was carried out in a specially fabricated glass assembly (Figure 2). The reaction cell was 6.6 cm in inner diameter and 9.0 cm in height with a total capacity of 305 mL. U-tube (2.0 cm outer diameter, 20 cm length) was fused from the base of the cell. The height of the tube inside the cell was 4.0 cm from the basal plane. The whole assembly was kept in a thermostat maintained at room temperature (30ºC). The transport experiments were performed with 50 ml of 0.58 x 10-4 M Cu(II) solutions at 5 pH as source phase and 50 ml, 1.0 M HCl as receiving phase. The liquid membrane consisted of 75 mL of 1.167 x 10-4 M o-APCRCPD (2b) in iso-amyl alcohol. The amount of copper transported from the source phase to the receiving phase was measured by FAAS/ICP-AES. The transport data were the average of 5 runs with an experimental error of less than 1.8%.
3. Results and Discussion
- In order to obtain the optimum conditions for the maximum extraction efficiency of Cu(II) with the azocalix[4]pyrrole dyes (1a-d, 2a-d), different factors affecting this process have been studied.
3.1. Solvent Extraction and Spectrophotometric Determination of Cu(II) with Azocalix[4] Pyrrole Dye
3.1.1. Spectral Characteristics of Cu(II)-Azocalix[4]- Pyrrole Dyes Complex
- The synthesized azocalix[4]pyrrole dyes (1a-d, 2a-d) were used for the extraction and spectrophotometric determination of Cu(II). It has been seen that among all the synthesized azocalix[4]pyrrole dyes (1a-d, 2a-d), o-amino phenol based meso-tetra(methyl)meso-tetra(3,5–dihydroxy phenyl) calix[4]pyrrole dye [2b (o-APCRCPD)] was the most sensitive reagent for the Cu(II) ion (Table 1). The complexation of Cu(II) with o-APCRCPD was studied under optimum conditions like pH, solvent, and reagent (o-APCRCPD) concentration. The maximum absorbance of the pinkish-red colored complex was measured at 540 nm and showed a bothochromic shift of 110 nm [540-430nm] from that of the reagent blank (Figure 3). The molar absoptivity was found to be 7048 L mol-1 cm-1. The system obeys Beer’s law in the range of 0.5-10 ug.mL-1.
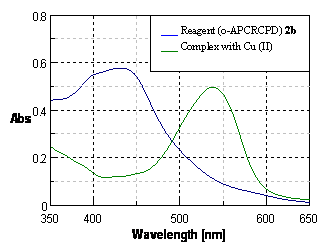 | Figure 3. Comparative spectra of reagent (o-APCRCPD) 2b (1.167x 10-4 M) and its Cu(II) complex in iso-amyl alcohol |
3.1.2. Effect of Variable on Extraction
- The complexation of Cu(II) with o-APCRCPD was studied under optimum conditions of pH, shaking time, solvent and regent (o-APCRCPD) concentration. Effect of pH and shaking time: The pH of the medium is a highly significant factor in extraction process. A series of experiments were carried out to study the effect of pH on the liquid-liquid extraction of Cu(II) with reagent, o-APCRCPD in iso-amyl alcohol. Maximum extraction of copper-complex was obtained in pH range of 4.5-5.0 (Figure 4); at the lower and higher pH the extraction was incomplete. This may be because the reagent remains protonated at pH lower than 4.5 and hydrolysis of Cu(II) ion at pH higher than 5.5. So, pH 5.0 was chosen for the extraction of Cu(II).
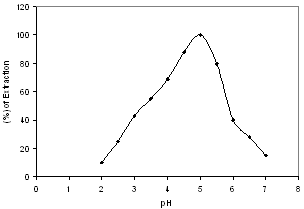 | Figure 4. Effect of pH on the extraction |
| |||||||||||||||||||||||||||||||||||||||
3.1.3. Stoichiometry of the Extracted Complex
- The composition of Cu(II) - o-APCRCPD complex extracted into iso-amyl alcohol has been studied by slope-ratio method[23] {plotting the graph of logarithm of distribution coefficient of the metal (logDm) against the negative logarithm of the reagent concentration -log (o-APCRCPD)}. The extraction is carried out by taking a fixed concentration of copper solution and varying amounts of reagent.
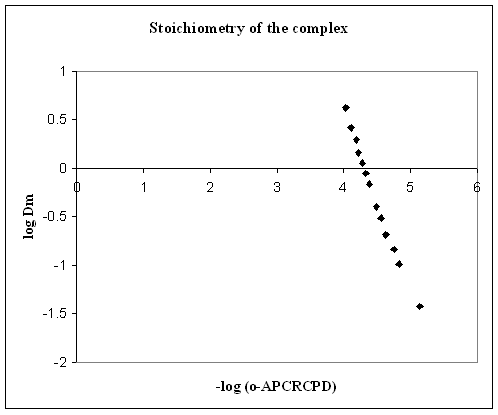 | Figure 5. Plot of log [Dm] against –log (o-APCRCPD) |
3.1.4. Effect of Diverse Ions
- To assess the usefulness of this method, the effect of foreign ions which often interfere on the extraction and spectrophotometric determination of Cu(II) were studied by adding a known amount of the metal-ion in to a solution containing 10 mL, 3 µg mL-1 of Cu(II) and the general procedure for the liquid-liquid extraction was followed. Cu(II) was extracted in the presence of several other ions to determine the sensitivity and utility of the method. Interference studies were performed by measuring the absorbance of the extracted organic-phase and aqueous phase by spectrophotometry/FAAS/ICP-AES. The tolerance limit was set as the amount of foreign ion causing a change of ± 0.02 in absorbance. It was observed that addition of 60-95 mg of Ca(II), Ag(I), Co(II), Ba(II), Zn(II), Mn(II), Pb(II), Ni(II), Hg(II), Cd(II), UO2(II), Fe(III), Ga(III), La(III), Th(IV), Ce(IV) and Sr(II) do not interfere in the determination of Cu(II). The results are shown in Table 3.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.2. Liquid Membrane Transport Studies
- A liquid membrane is a liquid phase, which separates two other liquid phases with which it is immiscible. These liquid membranes are of interest for both, possible technological application and fundamental studies of the transport process. The transport of Cu (II) through the membrane containing 1.167 X 10-4 M, o-APCRCPD from source phase containing 0.58 X 10-4 M, Cu (II) to the receiving phase of 1.0 M HCl was carried out. As evident from the Figure 6 the concentration of Cu(II) in the source phase started to decrease continuously and it took about 28 min to transport half of the Cu(II) from source phase to receiving phase (curve A). On the other hand, concentration of Cu (II) in the receiving phase starts increasing as shown in the curve B.
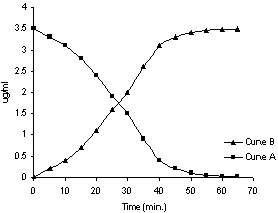 | Figure 6. Transport profile of Cu(II) through the liqiud membrane containing o-APCRCPD at 30ºC |
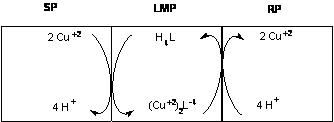 | Figure 7. Proposed mechanism for transport of Cu(II) through a liquid membrane containing o-APCRCPD (SP: Source Phase; LMP: Liquid membrane Phase; RP: Receiving Phase) |
| ||||||||||||||||||||||||||||||||||||||
3.3. Preconcentration of Cu(II)
- The concentration of Cu(II) in natural water is too low for its direct determination. Therefore, preconcentration or enrichment step is necessary to bring the sample to the detectable limits of existing instrumental methods. The method was studied for the preconcentration of copper in terms of its preconcentration factors.PF=Concentration of metal in organic solution/Initial concentration of metal in aqueous solution.The preconcentration study was carried out by extracting 40 μg of Cu (II) in 1000 mL aqueous phase with 6 mL of 0.015% o-APCRCPD in iso-amyl alcohol. To evaluate the efficiency of preconcentration, expressed as recovery, the concentration of Cu (II) in organic phase and the aqueous phase was determined by spectrophotometry/ FAAS/ICP- AES respectively. Quantitative determination was possible with recovery up to 97% with a concentration factor of 120.
3.4. Analytical Applications
- To investigate the applicability of the recommended procedure to natural water samples (taken from different locations of Ahmedabad city, Gujarat, India), the recoveries of known amounts of Cu (II) added to these samples were examined. The results obtained are given in Table 4. Spectrophotometric determination of Cu (II) gave satisfactory results when compared to those obtained by ICP-AES.
4. Conclusions
- Out of eight azocalix[4]pyrrole dyes, prodigious resorcinarenecalix[4]pyrrole 2b was found to be an effective reagent for the liquid-liquid extraction, preconcentration and transport of Cu(II) across a liquid membrane in iso-amyl alcohol. This reagent bearing a calixpyrrole ring, which are known anion sensors, holds great potential for their use as ditopic receptors[24] and the work is under progress in our lab. The described method is simple, sensitive and specific for the determination of copper ion in presence of large quantities of associated metal ions. The results obtained from the analysis of natural water samples establish the reliability, simplicity and robustness of the method.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the financial assistance provided by CSIR, New Delhi, India to carry out this work. The authors also acknowledge CDRI (Lucknow), SAIF (IIT, Mumbai) and CSMCRI (Bhavnagar) for providing instrumental facilities and INFLIBNET, Ahmedabad for providing online journals.
References
| [1] | Z. Asfari, V. Bohmer, J. Harrowfield, and Vicens J. Calixarene 2001 (Kluwer Academic Publishers: Netherlands, 2001) |
| [2] | C.D. Gutsche. Calixarenes Revisited; Monographs in Supramolecular Chemistry Ed.; J. F. Stoddart (RSC, Cambridge, 1998), p. 149-167 |
| [3] | V. Bohmer, and J. Vicens. Calixarenes; A Versatile Class of Macrocyclic Compounds (Kluwer Academic Publishers, Dordrecht, 1991), p. 127-171 |
| [4] | (a) F. Oueslati, I. Dumazeat-Bonnamour, and R. lamartine, New J. Chem. 27, 644 (2003). (b) O.O. Karakus, and H. Deligoz, J. of Incl. Phenom. Macrocycl. Chem. (2008) DOI: 10.1007/s10847-008-9421-9. (c) H. Deligoz, J. of Incl. Phenom. Macrocycl. Chem. 55, 197 (2006) |
| [5] | (a) O. Manabe, K. Asakura, T. Nishi, and S. Shinkai, Chem. Lett. 1219 (1990). (b) V.K. Jain, and P.H. Kanaiya, J. of Incl. Phenom. Macrocycl. Chem. 62(1-2), 111, (2008) |
| [6] | (a) P. Lhotak, J. Moravek, and I. Stibor, Tetrahedron Lett. 43, 3665 (2002). (b) F. Oueslati, I. Dumazet-Bonnamour, and R. Lamartine, Supramolecular Chemistry 17(3), 227 (2005) |
| [7] | (a) A. Skwierawska, J.F. Biernat, and R. Bilewicz, Supramolecular Chemistry 12(2), 213 (2000). (b) E. Luboch, E. Wagner-Wysiecka, M. Fainerman-Melnikova, L.F. Lindoy, and J.F. Biernat, Supramolecular Chemistry 18(7), 593 (2006). (c) U. Harikrishnan, and S.K. Menon, Dyes and Pigments 77(2), 462 (2008) |
| [8] | (a) R. Gu, S. Depretere, J. Koteck, J. Budka, E. Wangner-wysiecka, J.E. Biernat, and W. Dehaen, Org. Biomol. Chem. 3, 2921 (2005). (b) R. Nishiyabu, M.A. Palacious, W.P. Dehaen, and P. Jr. Anzenbacher, J. Am. Chem. Soc. 128, 11496 (2006). (c) R. Nishiyabu, M.A. Palacious, W.P. Dehaen, and P.Jr. Anzenbacher, J. Am. Chem. Soc. 128, 11496 (2006). (d) S. M. S. Chauhan, B. Garg, and T. Bishat, Supramolecular Chemistry 21(5), 394 (2009). (e) V. K. Jain, H. C. Mandalia, H. S. Gupte, and D. J. Vyas, Talanta 79, 1331 (2009). (f) V. K. Jain, H. C. Mandalia, and N. Bhojak, Fibers and Polymers 11(3), 363 (2010). (g) B. Garg, T. Bishat, and SMS Chauhan, New Journal of Chemistry 34(7), 1251 (2010) |
| [9] | (a) H.W. Nurnberg, Pollutants and their ecotoxicological significance (Wiley, Chichester, 1985). (b) D. Purves, Trace element contamination of the environments (Elsevier Science Publishers B. V., Netherlands, 1985) |
| [10] | (a) W.Y. Sang, H.F. Zhao, T.X. Zhang, M.G. Tan, G.S. Zhuang, B.L. Chen, Z.Z. Huang, H.L. Tian, and Q.F. Sun, Biol. Trace Elem. Res. 71, 617 (1999). (b) M. Soylak, and M. Dogan, Environ. Bull. 4, 35 (1995). (c) R. Saraymen, M. Soylak, L. Elci, and M. Dogan, Environ. Bull. 6, 694 (1997). (d) Q. Yang, K. Desmit, and J. Smeyersverbeke, J. Agr. Food Chem. 43, 2652 (1995). (e) R. Saraymen, M. Soylak, and I. Narin, Environ. Bull. 7, 403 (1998) |
| [11] | (a) Z.W. Zhang, S. Shimbo, N. Ochi, M. Eguchi, T. Watanabe, C.S. Moon, and M. Ikeda, Sci. Total Environ. 205, 179 (1997). (b) E.J. Gawalko, T.W. Nowicki, J. Babb, R. Tkachuk, and S.L. Wu, J. AOAC Int. 80, 379 (1997). (c) S.S. Chen, C.M. Chen, C.C. Cheng, and S.S. Chou, J. Food Drug. Anal. 7, 207 (1999). (d) M.T. Vidal, M.C. Pascual-Marti, A. Salvador, and C. Llabata, Microchem. J. 72, 221 (2002). (e) Y.C. Chen, and M.H. Chen, J. Food Drug. Anal. 9, 107 (2001). (f) D. Sancho, L. Deban, I. Campos, R. Pardo, and M. Vega, Food Chem. 71, 139 (2000). (g) Y.M. Hsu, J.M. Hwang, and T.R. Yeh, J. Food Drug Anal. 9, 178 (2001) |
| [12] | (a) M.H. Freemantle, Chemistry in Action (Macmillan Education Ltd., London, 1989). (b) M.E. Khalifa, M.A. Akl, and S.E. Ghazy, Chem. Pharm. Bull. 49, 664 (2001). (c) C.N. Sawyer, P.L. McCarty, and G.F. Parkin, Chemistry for Environmental Engineering 4th edition (New York: McGraw-Hill, 1996) |
| [13] | S.E. Ghazy, S.E. Samra, A-F.M. Mahdy, and S.M. El-Morsy, Anal. Sci. 19, 1401 (2003) |
| [14] | (a) Y. Goksungur, S. Uren, and U. Guvenc, Bioresource Technol. 96, 103 (2005). (b) J.S. Kim, S. Akeprathumchia, and S. Wickramasinghe, J. Membr. Sci. 182, 161 (2001). (c) C. Blocher, J. Dorda, V. Mavrov, H. Chmiel, N.K. Lazaridis, and K.A. Matis, Water Res. 37, 4018 (2003) |
| [15] | D.E. Leyden, and W. Wegscheider, Anal. Chem. 53, 1059 (1981) |
| [16] | (a) J. Di, Y. Wu, and Y. Ma, Spectrochim. Acta A 61(5), 937 (2005). (b) M. Yaman, and I. Akdeniz, Anal. Sci. 20(9), 1363 (2004). (c) J.Y. Cabon, Spectrochim. Acta B 57(5), 939 (2002). (d) M.D. Ioannidou, G.A. Zachariadis, A.N. Anthemidis, and J.A. Stratis, Talanta 65(1), 92 (2005). (e) Z. Kowalewska, A. Ruszczynska, and E. Bulska, Spectrochim. Acta B 60(3), 351 (2005). (f) H. Kim, and H. Choi, Talanta 55(1), 163 (2001) |
| [17] | (a) K.Z. Brainina, N.Y. Stozhko, G.M. Belysheva, O.V. Inzhevatova, L.I. Kolyadina, C. Cremisini, and M. Galletti, Anal. Chim. Acta 514(2), 227 (2004). (b) C. Lu, J. Lin, C.W. Huie, and M. Yamada, Anal. Sci. 19(4), 557 (2003) |
| [18] | S.E. Ghazy, and G.A.E. Mostafa, Egypt J. Chem. 45, 855 (2002) |
| [19] | (a) T. Tilki, I. Sener, F. Karci, A. Gulce, and H. Deligoz, Tetrahedron 61, 9624 (2005). (b) L. Lua, S. Zhub, X. Liua, Z. Xiec, and X. Yanc, Analytica Chimica Acta 535, 183 (2005) |
| [20] | V.K. Jain, and H.C. Mandalia, J. Incl. Phenom. Macrocycl. Chem. 63, 27 (2009) |
| [21] | J.A. Dean, Lange’s Handbook of Chemistry 15th Edition (McGraw-Hill: New York, 1999) |
| [22] | (a) Z. Marczenko, Spectrophotometric Determination of Elements (Ellis-Horwood Ltd: Chichester, 1976). (b) G.H. Jeffery, J. Bassett, J. Mendham, and R.C. Denny, Vogel’s Textbook of Chemical Analysis 5th Edition (Wiley: New York, 1989) |
| [23] | T.B. Barnko, and O.W. Jerome, Anal. Chem. 45, 1519 (1973) |
| [24] | (a) V. K. Jain, and H. C. Mandalia, Heterocycles 71(6), 1261 (2007). (b) V. K. Jain, H. C. Mandalia, and E. Suresh, J. of Inclusion Phenomena and Macrocyclic Chemistry 62, 167 (2008) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML