| [1] | Tristan Jaillard, Michael Roger, Anne Galinier, Pascale Guillou, Alexandre Benani, Corinne Leloup, Louis Casteilla, Luc Pénicaud, Anne Lorsignol, “Hypothalamic reactive oxygen species are required for insulin-induced food intake inhibition: An NADPH oxidase-dependent mechanism”, Diabetes, vol.58,no.7, pp.1544-1549, 2009. |
| [2] | Jackob Moskovitz, Moon Bin Yim, P Boon Chock, “Free radicals and disease”, Archives of Biochemistry and Biophysics, vol.397,no.2, pp.354-359, 2002. |
| [3] | Earl R Stadtman, “Protein oxidation and aging”, Free Radical Research, vol.40,no.12, pp.1250-1258, 2006. |
| [4] | Agnes W. Boots, Guido R.M.M. Haenen, Aalt Bast, Health effects of quercetin: From antioxidant to nutraceutical. European Journal of Pharmacology, vol.585, no.2-3,pp. 325-337, 2008. |
| [5] | Fátima Paiva-Martins, Rui Correia, Susana Félix, Pedro Ferreira, Michael H Gordon, “Effects of enrichment of refined olive oil with phenolic compounds from olive leaves”, Journal of Agricultural and Food Chemistry, vol.55,no.10, pp.4139-4143, 2007. |
| [6] | M A Soobrattee, V S Neergheen, A Luximon-Ramma, O I Aruoma, T Bahorun, “Phenolics as potential antioxidant therapeutic agents: Mechanism and actions” Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, vol.579,no.1-2, pp.200-213, 2005. |
| [7] | Dong-Young Choi, Young-Jung Lee, Jin Tae Hong, Hwa-Jeong Lee, “Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease”, Brain Research Bulletin, vol.87,no.2-3, pp.144-153, 2012. |
| [8] | Andrés Moure, Jose M. Cruz, Daniel Franco, J Manuel Domı́nguez, Jorge Sineiro, Herminia Domı́nguez, Marı́a José Núñez, J Carlos Parajó, “Natural antioxidants from residual sources”, Food Chemistry, vol.72, no.2, pp.145-171, 2001. |
| [9] | Nagendran Balasundram, Kalyana Sundram, Samir Samman, “Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses”, Food Chemistry, vol.99, no.1, pp.191-203, 2006. |
| [10] | Enma Conde, Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, “Fractionation of antioxidants from autohydrolysis of barley husks”, Journal of Agricultural and Food Chemistry, vol.56,no.22, pp.10651-10659, 2008. |
| [11] | Gargi Dey, Ashish Sachan, Shashwati Ghosh, Adinpunya Mitra, “Detection of major phenolic acids from dried mesocarpic husk of mature coconut by thin layer chromatography” Industrial Crops and Products, vol.18,no.2, pp.171-176, 2003. |
| [12] | Sweetie R Kanatt, Ramesh Chander, P Radhakrishna, Arun Sharma, ”Potato peel extract- a natural antioxidant for retarding lipid peroxidation in radiation processed lamb meat”, Journal of Agricultural and Food Chemistry, vol.53,no.5, pp.1499-1504, 2005. |
| [13] | Aleksandra Szydłowska-Czerniak, Konrad Trokowski, Edward Szłyk, “Optimization of extraction conditions of antioxidants from sunflower shells (Helianthus annuus L.) before and after enzymatic treatment”, Industrial Crops and Products, vol.33,no.1, pp.123-131, 2011. |
| [14] | K Nagendra Prasad, Bao Yang, Shaoyu Yang, Yulong Chen, Mouming Zhao, Muhammad Ashraf, Yueming Jiang, “Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn) seeds”, Food Chemistry, vol.116, no.1,pp.1-7, 2009. |
| [15] | Theodora-Ioanna Lafka, Andriana E Lazou, Vassilia J Sinanoglou, Evangelos S Lazos, “Phenolic and antioxidant potential of olive oil mill wastes”, Food Chemistry, vol.125,no.1,pp.92-98, 2011. |
| [16] | M Herrero, M Castro-Puyana, L Rocamora-Reverte, J A Ferragut, A Cifuentes, E Ibáñez, “Formation and relevance of 5-hydroxymethylfurfural in bioactive subcritical water extracts from olive leaves” Food Research International, vol.47,no.1, pp.31-37, 2012. |
| [17] | María Luisa Soto, Enma Conde, Noelia González-López, María Jesús Conde, Andrés Moure, Jorge Sineiro, Elena Falqué, Herminia Domínguez, María José Núñez, Juan Carlos Parajó, “Recovery and concentration of antioxidants from winery wastes”, Molecules, vol.17,no.3, pp.3008-3024, 2012. |
| [18] | Belén Suárez, Ángel L Álvarez, Yolanda Diñeiro García, Gloria del Barrio, Anna Picinelli Lobo, Francisco Parra, “Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace” Food Chemistry, vol.120,no.1,pp.339-342, 2010. |
| [19] | Shinichi Someya, Yumiko Yoshiki, Kazuyoshi Okubo, “Antioxidant compounds from bananas (Musa cavendish)”, Food Chemistry, vol.79, no.3, pp.351-354, 2002. |
| [20] | Shela Gorinstein, Olga Martin-Belloso, Antonin Lojek, Milan Číž, Robert Soliva-Fortuny, Yong-Seo Park, Abraham Caspi, Imanual Libman, Simon Trakhtenberg, "Comparative content of some phytochemicals in Spanish apples, peaches, and pears", Journal of the Science of Food and Agriculture, vol.82,no.10, pp.1166-1170, 2002. |
| [21] | Nicolai Berardini, Matthias Knödler, Andreas Schieber, Reinhold Carle, “Utilization of mango peels as a source of pectin and polyphenolics” Innovative Food Science and Emerging Technologies, vol.6, no.1,pp.442-452, 2005. |
| [22] | Miguel Herrero, Alejandro Cifuentes, Elena Ibañez. “Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review”, Food Chemistry, vol.98, no.1, pp.136-148, 2006. |
| [23] | Xiaoping Yuan, Jing Wang, Huiyuan Yao, “Antioxidant activity of feruloylated oligosaccharides from wheat bran”, Food Chemistry, vol.90,no.4, pp.759-764, 2005. |
| [24] | Jing Wang, Baoguo Sun, Yanping Cao, Chengtao Wang, “Wheat bran feruloyl oligosaccharides enhance the antioxidant activity of rat plasma” Food Chemistry, vol.123,no.2, pp.472-476, 2010. |
| [25] | Jonas Lewin Bauer, Britta Harbaum-Piayda, Heiko Stöckmann, Karin Schwarz, “Antioxidant activities of corn fiber and wheat bran and derived extracts”, LWT - Food Science and Technology, vol.50,no.1,pp.132-138, 2013. |
| [26] | Huamao Yuan, Jinming Song, Weiwei Zhang, Xuegang Li, Ning Li, Xuelu Gao, “Antioxidant activity and cytoprotective effect of ĸ-carrageenan oligosaccharides and their different derivatives”, Bioorganic & Medicinal Chemistry Letters, vol.16,no.5, pp.1329-1334, 2006. |
| [27] | Baohua Kong, Youling L Xiong, “Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action”. Journal of Agricultural and Food Chemistry, vol.54,no.16, pp.6059-6068, 2006. |
| [28] | Xinyan Peng, Youling L. Xiong, Baohua Kong, “Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance”, Food Chemistry, vol.113,no.1, pp.196-201, 2009. |
| [29] | Guowan Su, Lin Zheng, Chun Cui, Bao Yang, Jiaoyan Ren, Mouming Zhao, “Characterization of antioxidant activity and volatile compounds of Maillard reaction products derived from different peptide fractions of peanut hydrolysate”, Food Research International, vol.44,no.10, pp.3250-3258, 2011. |
| [30] | Rong He, Xingrong Ju, Jian Yuan, Lifeng Wang, Abraham T. Girgih, Rotimi E. Aluko, “Antioxidant activities of rapeseed peptides produced by solid state fermentation” Food Research International, vol.49,no.1, pp.432-438, 2012. |
| [31] | Dejian Huang, Boxin Ou, Ronald L Prior, “The chemistry behind antioxidant capacity assays”, Journal of Agricultural and Food Chemistry, vol.53,no.6, pp.1841-1856, 2005. |
| [32] | Ryan Elias, Sarah Kellerby, Eric Decker, “Antioxidant activity of proteins and peptides”, Critical Reviews in Food Science and Nutrition, vol.48,no.5, pp.430-441, 2008. |
| [33] | C Sánchez-Moreno, “Methods used to evaluate the free radical scavenging activity in foods and biological systems”, Food Science and Technology International, vol.8,no.3, pp.121-137, 2002. |
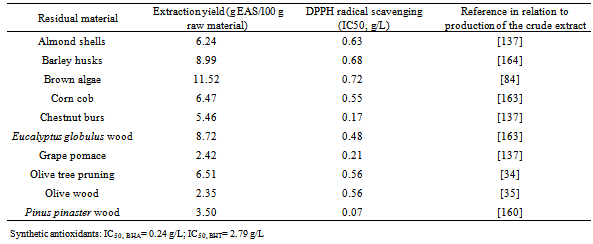
| [34] | Enma Conde, Cristobal Cara, Andrés Moure, Encarnación Ruiz, Eulogio Castro, Herminia Domínguez, “Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning”, Food Chemistry, vol.114, no. 3, pp.806-812. 2009. |
| [35] | Eulogio Castro, Enma Conde, Andrés Moure, Elena Falqué, Cristobal Cara, Encarnación Ruiz, Herminia Domínguez, “Antioxidant activity of liquors from steam explosion of Olea europea Wood”, Wood Science and Technology, vol.42,no.7, pp.579-592, 2008. |
| [36] | D Jiménez-Álvarez, F Giuffrida, P A Golay, C Cotting, A Lardeau, Brendan J Keely, “Antioxidant activity of oregano, parsley, and olive mill wastewaters in bulk oils and oil-in-water emulsions enriched in fish oil”, Journal of Agricultural and Food Chemistry, vol.56,no.16, pp.7151-7159, 2008. |
| [37] | D A Pereira de Abreu, P Paseiro Losada, J Maroto, J M Cruz, “Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.)”, Food Research International, vol.43,no.5, pp.1277-1282, 2010. |
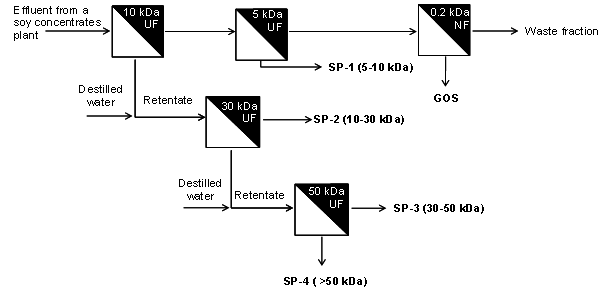
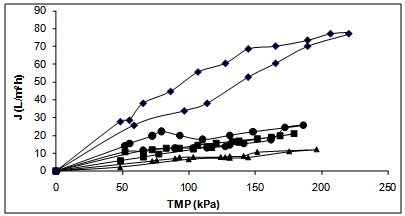
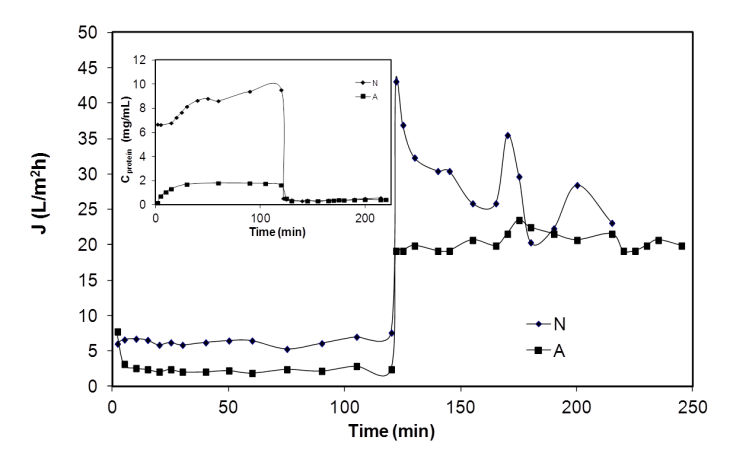
| [38] | Andres Moure, Herminia Domínguez, Juan Carlos Parajó, “Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates”, Process Biochemistry, vol.41,no.2, pp.447-456, 2006. |
| [39] | Chen, H. M., Muramoto, K., Yamauchi, F., Fujimotom K., Nokiharam K. “Antioxidative properties of histidine - containing peptides designed from peptide fragments found in the digests of a soybean protein”, Journal of Agricultural and Food Chemistry, vol.46,no. ,pp.49-53, 1998. |
| [40] | Tao Zhang, Yanhong Li, Ming Miao, Bo Jiang, “Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates” Food Chemistry, vol.128,no.1, pp.28-33, 2011. |
| [41] | Dong-Jiann Huang, Hsien-Jung Chen, Wen-chi Hou, Chun-Der Lin, Yaw-Huei Lin, “Active recombinantthioredoxin h protein with antioxidant activities from sweet potato (Ipomoea batatas[L.] Lam Tainong storage roots”, Journal of Agricultural and Food Chemistry, vol.52,no.15, pp.4720-4724, 2004. |
| [42] | E Aida Peña-Ramos, Youling L Xiong, “Whey and soy protein hydrolysates inhibit lipid oxidation in cooked pork patties”, Meat Science, vol.64,no.3, pp.259-263, 2003. |
| [43] | Szu Kai Chen, Min Lang Tsai, Jin Ru Huang, Rong Huei Chen, "In vitro antioxidant activities of low-molecular-weight polysaccharides with various functional groups", Journal of Agricultural and Food Chemistry, vol.57,no.7, pp.2699-2704, 2009. |
| [44] | Ella Allerdings, John Ralph, Hans Steinhart, Mirko Bunzel, “Isolation and structural identification of complex feruloylated heteroxylan side-chain from maize bran”, Phytochemistry, vol.67, no.12, pp.1276-1286, 2006. |
| [45] | Petros Katapodis, Maria Vardakou, Emanuel Kalogeris, Dimitris Kekos, Basil J. Macris Paul Christakopoulos, “Enzymic production of a feruloylated oligosaccharide with antioxidant activity from wheat flour arabinoxylan”, European Journal of Nutrition, vol.42,no1, pp.55-60, 2003. |
| [46] | Devin J Rose, George E Inglett, “Two-stage hydrothermal processing of wheat (Triticum aestivum) bran for the production of feruloylated arabinoxylooligosaccha-rides”, Journal of Agricultural and Food Chemistry, vol.58,no.10, pp.6427-6432, 2010. |
| [47] | Xiaoping Yuan, Jing Wang, Huiyuan Yao, Feng Chen, “Free radical-scavenging capacity and inhibitory activity on rat erythrocyte hemolysis of feruloyl oligosaccharides from wheat bran insoluble dietary fiber”, LWT - Food Science and Technology, vol.38,no.8, pp.877-883, 2005. |
| [48] | Anna Ebringerová, Zdenka Hromádková, Vera Hříbalová, Chunlin Xu, Bjarne Holmbom, Anna Sundberg, Stefan Willför, “Norway spruce galactoglucomannans exhibiting immunomodulating and radical-scavenging activities”, International Journal of Biological Macromolecules, vol.42,no.1, pp.1-5, 2008. |
| [49] | Anna Ebringerová, Zdenka Hromádková, Zuzana Košt’álová, Vlasta Sasinková, "Chemical valorization of agricultural by-products: isolation and characterization of xylan-based antioxidants from almond shell biomass", BioResources, vol.3, no.1, pp. 60-70, 2008. |
| [50] | Z Hromádková, Z Košt’álová, A Ebringerová, “Comparison of conventional and ultrasound-assisted extraction of phenolics-rich heteroxylans from wheat bran”, Ultrasonics Sonochemistry, vol.15,no.6, pp.1062-1068, 2008. |
| [51] | Raniere Fagundes Melo-Silveira, Gabriel Pereira Fidelis, Mariana Santana Santos Pereira Costa, Cinthia Beatrice Silva Telles, Nednaldo Dantas-Santos, Susana de Oliveira Elias, Vanessa Bley Ribeiro, Afonso Luis Barth, Alexandre José Macedo, Edda Lisboa Leite, Hugo Alexandre Oliveira Rocha, “In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs”, International Journal of Molecular Sciences, vol.13,no.1, pp.409-426, 2012. |
| [52] | Jelena Bogdanović Pristov, Aleksandra Mitrović, Ivan Spasojević, “A comparative study of antioxidative activities of cell-wall polysaccharides”, Carbohydrate Research, vol.346,no.14, pp.2255-2259, 2011. |
| [53] | B R Veenashri, G Muralikrishna, “In vitro anti-oxidant activity of xylo-oligosaccharides derived from cereal and millet brans. A comparative study”, Food Chemistry, vol.126, no.3, pp.1475-1481, 2011. |
| [54] | Kyoji Yoshino, Naoki Higashi, Kunimasa Koga, “Antioxidant activities of acidic xylooligosaccharide”, Memoirs of Numazu College of Technology, vol.41, pp.103-105, 2007. |
| [55] | Hua Chen, Li-jun Liu, Jian-jun Zhu, Bo Xu, Rui Li, “Effect of soybean oligosaccharides on blood lipid, glucose levels and antioxidant enzymes activity in high fat rats”, Food Chemistry, vol.119, no.4, pp.1633-1636, 2010. |
| [56] | Hai-Min Chen, Xiao-Jun Yan, “Antioxidant activities of agaro-oligosaccharides with different degrees of polymerization in cell-based system” Biochimica et Biophysica Acta, vol.1722, no.1, pp.103-111, 2005. |
| [57] | Li Xu, Shiyong Wang, “The Ginkgo biloba extract concentrated by nanofiltration”, Desalination, vol.184, no.1-3, pp.305-313, 2005. |
| [58] | Jing Li, Howard A Chase, “Applications of membrane techniques for purification of natural products. Biotechnology Letters, vol.32, no.5 pp.601–608, 2010. |
| [59] | Aureanna Nairne Negrão Murakami, Renata Dias de Mello Castanho Amboni, Elane Schwinden Prudêncio, Edna Regina Amante, Laura de Moraes Zanotta, Marcelo Maraschin, José Carlos Cunha Petrus, Reinaldo Francisco Teófilo, “Concentration of phenolic compounds in aqueous mate (Ilex paraguariensis A. St. Hil) extract through nanofiltration”, LWT - Food Science and Technology, vol.44,no.10, pp.2211-2216, 2011. |
| [60] | Marcel Mulder, “Basic principles of membrane technology”. Kluwer academic publishers, Dordrecht, The Netherlands, pp564, 1996 |
| [61] | Cesar de Morais Coutinho, Ming Chih Chiu, Rodrigo Correa Basso, Ana Paula Badan Ribeiro, Lireny Aparecida Guaraldo Gonçalves, Luiz Antonio Viotto, “State of art of the application of membrane technology to vegetable oils: A review”, Food Research International, vol.42,no.5-6, pp.536–550, 2009. |
| [62] | Munir Cheryan, Ultrafiltration and microfiltration handbook. Technomic Publishing Co., Lancaster, PA, p527, 1998. |
| [63] | Richard W. Baker, Membrane Technology and Applications. John Wiley & Sons, Ltd. p545, 2004. |
| [64] | Edoardo Turano, Stefano Curcio, Maria G De Paola, Vincenza Calabrò, Gabriele Iorio, "An integrated centrifugation–ultrafiltration system in the treatment of olive mill wastewater", Journal of Membrane Science, vol.209,no.2, pp.519-531, 2002. |
| [65] | Maria Diná Afonso, Javier Ferrer, Rodrigo Bórquez, “An economic assessment of proteins recovery from fish meal effluents by ultrafiltration”, Trends in Food Science & Technology, vol.15, no.10, pp.506–512, 2004. |
| [66] | Ana P. Aguiar Prudêncio, Elane Schwinden Prudêncio, Renata D.M. Castanho Amboni, Aureanna N. Negrão Murakami, Marcelo Maraschin, José C. Cunha Petrus, Paulo José Ogliari, Rodrigo Santos Leite, “Phenolic composition and antioxidant activity of the aqueous extract of bark from residues from mate tree (Ilex paraguariensis St. Hil.) bark harvesting concentrated by nanofiltration. Food and Bioproducts Processing, vol.90,no.3, pp.399-405, 2012. |
| [67] | G Paun, E Neagu, A Tache, G L Radu, V Parvulescu, “Application of the nanofiltration process for concentration of polyphenolic compounds from Geranium robertianum and Salvia officinalis extracts”, Chemical and Biochemical Engineering Quarterly, vol.25, no.4, pp.453-460, 2011. |
| [68] | M R Sohrabi, S S Madaeni, M Khosravi, A M Ghaedi, M R Sohrabi, S S Madaeni, M Khosravi, A M Ghaedi, "Separation and Purification Technology", vol.75,no.2, pp.121-126, 2010. |
| [69] | Beatriz Díaz-Reinoso, Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, "Membrane concentration of antioxidants from Castanea sativa leaves aqueous extracts", Chemical Engineering Journal, vol.175, pp.95-102, 2011. |
| [70] | Zsuzsanna Molnár, Kübra Özdemir, Erika Békássy-Molnár, Gyula Vatai, “Valuable components concentration of raspberry marc extract by membrane filtration” in Proceedings of 6th International CIGR Technical Symposium - Towards a Sustainable Food Chain: Food Process, Bioprocessing and Food Quality Management”, 2011. |
| [71] | Bartosz Tylkowski, Boryana Trusheva, Vassya Bankova, Marta Giamberini, Georgi Peev, Agnessa Nikolova, "Extraction of biologically active compounds from propolis and concentration of extract by nanofiltration", Journal of Membrane Science, vol.348,no.1-2, pp.124-130. 2010. |
| [72] | Beatriz C B S Mello, José Carlos Cunha Petrus, Miriam Dupas Hubinger, “Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nanofiltration”, Journal of Food Engineering, vol.96,no,4, pp.533-539, 2010. |
| [73] | Bartosz Tylkowski, Iren Tsibranska, Ruslan Kochanov, Georgi Peev, Marta Giamberini, “Concentration of biologically active compounds extracted from Sideritis ssp. L. by nanofiltration”, Food and Bioproducts Processing, vol.89, no.4, pp.307-314, 2011. |
| [74] | D Peshev, L G Peeva, G Peev, I I R Baptista, A T Boam, “Application of organic solvent nanofiltration for concentration of antioxidant extracts of rosemary (Rosmarinus officiallis L.)”, Chemical Engineering Research and Design, vol.89, no.3, pp.318-327, 2011. |
| [75] | Haseeb Nawaz, John Shi, Gauri S. Mittal, Yukio Kakuda, "Extraction of polyphenols from grape seeds and concentration by ultrafiltration", Separation and Purification Technology, vol.48,no.2, pp176-181, 2006. |
| [76] | Beatriz Díaz-Reinoso, Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, “Ultra- and nanofiltration of aqueous extracts from distilled fermented grape pomace”, Journal of Food Engineering, vol.91, no.4, pp.587-593, 2009. |
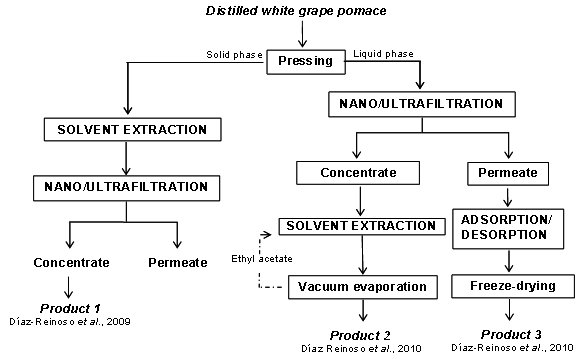
| [77] | L M Cheung, P C K Cheung, “Mushroom extracts with antioxidant activity against lipid peroxidation”. Food Chemistry, vol.89,no.3, pp.403-409, 2005. |
| [78] | Zer-Ran Yu, Chien-Ching Hung, Yih-Ming Weng, Chun-Li Su, Be-Jen Wang, “Physiochemical, antioxidant and whitening properties of extract from root cortices of mulberry as affected by membrane process”, LWT - Food Science and Technology, vol.40,no.5, pp.900-907, 2007. |
| [79] | Hai-Feng Gu, Chun-Mei Li, Yu-Juan Xu, Wan-Feng Hu, Mei-Hong Chen, Qiong-Hong Wan, “Structural features and antioxidant activity of tannin from persimmon pulp”, Food Research International, vol.41,no.2, pp.208-217, 2008. |
| [80] | Ahmad Kalbasi, Luis Cisneros-Zevallos, “Fractionation of monomeric and polymeric anthocyanins from Concord grape (Vitis labrusca L.) juice by membrane ultrafiltration”, Journal of Agricultural and Food Chemistry, vol,55,no.17, pp.7036-7042, 2007. |
| [81] | B Santamaría, G Salazar, S Beltrán, J L Cabezas, “Membrane sequences for fractionation of polyphenolic extracts from defatted milled grape seeds”, Desalination, vol.148,no.1-3, pp.103-109, 2002. |
| [82] | Marin Prodanov, Ignacio Garrido, Visitación Vacas, Rosa Lebrón-Aguilar, Montserrat Dueñas, Carmen Gómez - Cordovés, Begoña Bartolomé, “Ultrafiltration as alternative purification procedure for the characterization of low and high molecular-mass phenolics from almond skins”, Analytica Chimica Acta, vol.609,no.2, pp.241-251. 2008. |
| [83] | Beatriz Díaz-Reinoso, Noelia González-López, Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, “Recovery of antioxidants from industrial waste liquors using membranes and polymeric resins”, Journal of Food Engineering, vol.96,no.1, pp.127-133, 2010. |
| [84] | Noelia González-López, Andrés Moure, Herminia Domínguez, Juan C Parajó “Valorization of chestnut husks by non-isothermal hydrolysis”, Industrial Crops and Products, vol.36,no.1, pp.172-176, 2012. |
| [85] | Luiz A V Sarmento, Ricardo A F Machado, José C C Petrus, Thaisa R Tamanini, Ariovaldo Bolzan, “Extraction of polyphenols from cocoa seeds and concentration through polymeric membranes”, The Journal of Supercritical Fluids, vol.45,no.1, pp.64-69, 2008. |
| [86] | S Todisco, P Tallarico, B B Gupta, "Mass transfer and polyphenols retention in the clarification of black tea with ceramic membranes", Innovative Food Science and Emerging Technologies, vol.3,no.3, pp.255-262, 2002. |
| [87] | Ping Li, Yanhui Wang, Runyu Ma, Xiaolin Zhang, “Separation of tea polyphenol from Green Tea leaves by a combined CATUFM-adsorption resin process”, Journal of Food Engineering, vol.67, no.3, pp.253-260, 2005. |
| [88] | Zhihong Xu, Lei Li, Fawen Wu, Shujuan Tan, Zhibing Zhang, "The application of the modified PVDF ultrafiltration membranes in further purification of Ginkgo biloba extraction", Journal of Membrane Science, vol.255,no.1-2, pp.125-131, 2005. |
| [89] | Claudio Russo, "A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW)", Journal of Membrane Science, vol.288, no.1-2, pp.239-246, 2007. |
| [90] | Charis M. Galanakis, Eva Tornberg, Vassilis Gekas, “Clarification of high-added value products from olive mill wastewater”, Journal of Food Engineering, vol.99,no.2, pp.190-197, 2010. |
| [91] | E García-Castello, A Cassano, A Criscuoli, C Conidi, E Drioli, “Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system”, Water research, vol.44,no.13, pp3883-3892, 2010. |
| [92] | A Cassano, C Conidi, E Drioli, “Comparison of the performance of UF membranes in olive mill wastewaters treatment”. Water Research vol.45, no.10, pp.3197-3204, 2011. |
| [93] | Marisa Bernardo, Ana Santos, Paula Cantinho, Miguel Minhalma, “Cork industry wastewater partition by ultra/nanofiltration: A biodegradation and valorisation study”. Water Research, vol.45, no.2, pp.904-912, 2011. |
| [94] | Lei Xu, Karen Lamb, Linda Layton, Ashwani Kumar, “A membrane-based process for recovering isoflavones from a waste stream of soy processing” Food Research International, vol.37,no.9, pp867-874, 2004. |
| [95] | Yuanfeng Wang, Zhiwei Yang, Xinlin Wei, “Antioxidant activities potential of tea polysaccharide fractions obtained by ultrafiltration”, International Journal of Biological Macromolecules, vol.50,no.3, pp.558- 564, 2012. |
| [96] | Ruizhan Chen, Yuan Li, Hang Dong, Zhiqiang Liu, Shizhe Li, Simin Yang, Xinlong Li, “Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum caudatum Ait. and evaluation of its biological activities”, Ultrasonics Sonochemistry, vol.19,no.6, pp.1160-1168, 2012. |
| [97] | Hong Ye, Keqi Wang, Chunhong Zhou, Jun Liu, Xiaoxiong Zeng, “Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum”, Food Chemistry, vol.111,no.2, pp.428-432. 2008. |
| [98] | Jin-shui Wang, Mou-ming Zhao, Qiang-zhong Zhao, Yue-ming Jiang, “Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems” Food Chemistry, vol.101,no.4, pp.1658-1663, 2007. |
| [99] | Massimo Pizzichini, Claudio Russo, “Process for recovering the components of olive mill wastewater with membrane technologies”, WO 2005/123603, 2005. |
| [100] | Luigi Villanova, Luciano Villanova, Gianluca Fasiello, Alessandro Merendino, "Process for the recovery of tyrosol and hydroxytyrosol from oil mill wastewaters and catalytic oxidation method in order to convert tyrosol in hydroxytyrosol" EP1623960, 2008. |
| [101] | Maurizio Servili, Sonia Esposto, Gianluca Veneziani, Stefania Urbani, Agnese Taticchi, Ilona Di Maio, Roberto Selvaggini, Beatrice Sordini, GianFrancesco Montedoro, “Improvement of bioactive phenol content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment”, Food Chemistry, vol.124,no.4, pp.1308-1315, 2011. |
| [102] | Manuel Luís de Magalhaes Nunes da Ponte, José Luís Cardador dos Santos; Ana Alexandra Figueiredo Matias, Ana Vital Morgado Marques Nunes, Catarina Maria Martins Duarte, Joao Paulo Serejo Goulao Crespo. “Method of obtaining a natural hydroxytyrosol-rich concentrate from olive tree residues and subproducts using clean technologies”. WO 2007/013032 A2, 2007. |
| [103] | Janet Carrasco-Castilla, Alan Javier Hernández-Álvarez, Cristian Jiménez-Martínez, Carmen Jacinto-Hernández, Manuel Alaiz, Julio Girón-Calle, Javier Vioque, Gloria Dávila-Ortiz, “Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates”. Food Chemistry, vol.135,no.3, pp.1789-1795, 2012. |
| [104] | Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, “Ultrafiltration of industrial waste liquors from the manufacture of soy protein concentrates”, Journal of Chemical Technology and Biotechnology, vol.81, no.7, pp.1252-1258, 2006. |
| [105] | Anne Pihlanto, Sari Akkanen, Hannu J Korhonen, “ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum)”, Food Chemistry, vol.109,no.1, pp.104–112, 2008. |
| [106] | Zhengjun Xie, Junrong Huang, Xueming Xu, Zhengyu Jin, “Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate”, Food Chemistry, vol.111,no.2, pp.370-376, 2008. |
| [107] | Yumiko Yoshie-Stark, Yoshiko Wada, Andreas Wäsche, “Chemical composition, functional properties, and bioactivities of rapeseed protein isolates”, Food Chemistry, vol.107,no.1, pp.32-39, 2008. |
| [108] | Xiangzhen Kong, Huiming Zhou, Yufei Hua, Preparation and antioxidant activity of wheat gluten hydrolysates (WGHs) using ultrafiltration membranes. Journal of the Science Food and Agriculture, vol.88,no.5, pp.920-926, 2008. |
| [109] | Yichen Xia, Fatemeh Bamdad, Michael Gänzle, Lingyun Chen, “Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis”, Food Chemistry, vol.134, no.3, pp.1509-1518, 2012. |
| [110] | María Luisa Soto, Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, “Recovery, concentration and purification of phenolic compounds by adsorption: A review”, Journal of Food Engineering, vol.105,no.1, pp.1-27, 2011. |
| [111] | Judith Kammerer, Christian Schweizer, Reinhold Carle, Dietmar R. Kammerer, “Recovery and fractionation of major apple and grape polyphenols from model solutions and crude plant extracts using ion exchange and adsorbent resins”, International Journal of Food Science and Technology, vol.46,no.8, pp.1755-1767, 2011. |
| [112] | Judith Kammerer, Dietmar R Kammerer, Uwe Jensen, Reinhold Carle, “Interaction of apple polyphenols in a multi-compound system upon adsorption onto a food-grade resin”, Journal of Food Engineering, vol.96,no.4, pp.544-554, 2010. |
| [113] | Alfio Di Mauro, Biagio Fallico, Amedeo Passerini, Emanuele Maccarone, “Waste water from citrus processing as a source of hesperidin by concentration on styrene-divinylbenzene resin”, Journal of Agricultural and Food Chemistry, vol.48,no.6, pp.2291-2295, 2000. |
| [114] | Chaoyang Ma, Guangjun Tao, JianTang, Zaixiang Lou, Hongxin Wang, Xiaohong Gu, Liming Hu, Menglong Yin, "Preparative separation and purification of rosavin in by macroporous adsorption resin", Separation and Purification Technology, vol.69,no.1, pp.22-28, 2009. |
| [115] | Wei Liu, Su Zhang, Yuan-Gang Zu, Yu-Jie Fu, Wei Ma, Dong-Yang Zhang, Yu Kong, Xiao-Juan Li, "Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins", Bioresource Technology, vol.101,no.12, pp.4667-4675, 2010. |
| [116] | Monica Scordino, M., Alfio Di Mauro, Amedeo Passerini, Emanuele Maccarone, “Selective recovery of anthocyanins and hydroxycinnamates from a byproduct of citrus processing”, Journal of Agricultural and Food Chemistry, vol.53,no.3, pp.651-658, 2005. |
| [117] | Knut O Strætkvern, Sissel Løkra, Morten Aa Olander, Allan Lihme, “Food-grade protein from industrial potato starch effluent recovered by an expanded bed adsorption process”, Journal of Biotechnology, 118S1, no.S1-S189, pp.33, 2005. |
| [118] | E Robens, K-F Krebs, K Meyer, K K Unger, “Standardization of sorption measurements and reference materials for dispersed and porous solids”, in Studies in Surface Science and Catalysis, vol.120, Part A, pp.95-116, 1999. |
| [119] | Tadashi Adachi, Eiji Isobe, “Fundamental characteristics of synthetic adsorbents intended for industrial chromatographic separations”, Journal of Chromatography A, vol.1036, no.1, pp.33-44, 2004. |
| [120] | Xiaotian Geng, Ping Ren, Guopei Pi, Rongfu Shi, Zhi Yuan, Chunhong Wang, "High selective purification of flavonoids from natural plants based on polymeric adsorbent with hydrogen-bonding interaction", Journal of Chromatography A, vol.1216,no.47, pp.8331-8338, 2009. |
| [121] | Monica Scordino, M., Alfio Di Mauro, Amedeo Passerini, Emanuele Maccarone, “Adsorption of flavonoids on resins: Cyanidin 3-Glucoside”, Journal of Agricultural and Food Chemistry, vol.52,no.7, pp.1965-1972, 2004. |
| [122] | Dietmar R Kammerer, Zaid S Saleh, Reinhold Carle, Roger A. Stanley, "Adsortive recovery of phenolic compounds from apple juice", European Food Research and Technology, vol.224,no.5, pp.605-613, 2007. |
| [123] | Qiushuang Wang, Tiejin Ying, Muhammad Muzammil Jahangir, Tianjia Jiang, “Study on removal of coloured impurity in soybean oligosaccharides extracted from sweet slurry by adsorption resins”, Journal of Food Engineering, vol.111,no.2, pp.386-393, 2012. |
| [124] | N Seeram, R Lee, M Hardy, D Heber, "Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry", Separation and Purification Technology, vol.41,no.1, pp.49-55, 2005. |
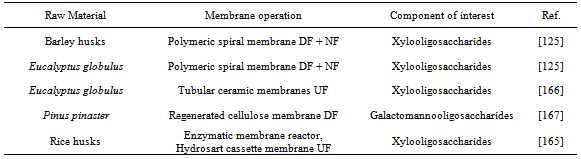
| [125] | Patricia Gullón, María Jesús González-Muñoz, Juan Carlos Parajó, "Manufacture and prebiotic potential of oligosaccharides derived from industrial solid wastes", Bioresource Technology, vol.102, no.10, pp.6112-6119, 2011. |
| [126] | Apostolis Agalias, Prokopios Magiatis, Alexios-Leandros Skaltsounis, Emmanuel Mikros, Anthony Tsarbopoulos, Evagelos Gikas, Ioannis Spanos, Thrasyvoulos Manios, “A new process for the management of olive oil mill waste water and recovery of natural antioxidants”, Journal of Agricultural and Food Chemistry, vol.55,no.7, pp.2671-2676, 2007. |
| [127] | Biao-Shi Wang, Bian-Sheng Li, Qing-Xiao Zeng, Hui-Xia Liu, “Antioxidant and free radical scavenging activities of pigments extracted from molasses alcohol wastewater” Food Chemistry, vol.107,no.3, pp.1198-1204, 2008. |
| [128] | Alfio Di Mauro, Elena Arena, Biagio Fallico, Amedeo Passerini, Emanuele Maccarone, "Recovery of anthocyanins from pulp wash of pigmented oranges by concentration on resins", Journal of Agricultural and Food Chemistry, vol.50,no.21, pp.5968-5974, 2002. |
| [129] | Navindra P Seeram, Lynn S Adams, Susanne M Henning, Yantao Niu, Yanjun Zhang, Muraleedharan G. Nair, David Heber, “In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice”, The Journal of Nutritional Biochemistry, vol.16,no.6, pp.360-367, 2005. |
| [130] | Elke Aehle, Sophie Raynaud-Le Grandic, Robert Ralainirina, Sylvie Baltora-Rosset, François Mesnard, Christophe Prouillet, Jean-Claude Mazière, Marc-André Fliniaux, “Development and evaluation of an enriched natural antioxidant preparation obtained from aqueous spinach (Spinacia oleracea) extracts by an adsorption procedure”, Food Chemistry, vol.86, no.4, pp.579-585, 2004. |
| [131] | Xiu-Lian Chang, Dong Wang, Bi-Yun Chen, Yong-Mei Feng, Shao-Hong Wen, Peng-Yuan Zhan, “Adsorption and desorption properties of macroporous resins for anthocyanins from the calyx extract of Roselle (Hibiscus sabdariffa L.)”, Journal of Agricultural and Food Chemistry, vol.60,no.9, pp.2368-2376, 2012. |
| [132] | Mi-Ryung Kim, Won-Chul Kim, Do-Youn Lee, Chan-Wha Kim, “Recovery of narirutin by adsorption on a non-ionic polar resin from a water-extract of Citrus unshiu peels”, Journal of Food Engineering, vol.78,no.1, pp.27-32, 2007. |
| [133] | Boqiang Fu, Jie Liu, Huan Li, Lei Li, Frank S C Lee, Xiaoru Wang, "The application of macroporous resins in the separation of licorice flavonoids and glycyrrhizic acid", Journal of Chromatography A, vol.1089,no.1-2, pp.18-24, 2005. |
| [134] | Yujie Fu, Yuangang Zu, Wei Liu, Chunlian Hou, Liyan Chen, Shuangming Li, Xiaoguang Shi, Meihong Ton,"Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins", Journal of Chromatography A, vol.1139,no.2, pp.206-213, 2007. |
| [135] | Sung Y Yoon, Won Jae Choi, Jong Moon Park, Ji-Won Yang, “Selective adsorption of flavonoid compounds from the leaf extract of Ginkgo biloba L”, “Biotechnology Techniques”, vol.11,no.8, pp.553-556, 1997. |
| [136] | Monica Scordino, Alfio Di Mauro, Amedeo Passerini, Emanuele Maccarone, “Adsorption of flavonoids on resins: Hesperidin”, Journal of Agricultural and Food Chemistry, vol.51,no.24, pp.6998-7004, 2003. |
| [137] | Enma Conde, Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, “Production of antioxidants by non-isothermal autohydrolysis of lignocellulosic wastes”, LWT - Food Science and Technology, vol.44,no.2, pp.436-442, 2011. |
| [138] | Gerald T Gourdin, Steven L Richheimer, Michael S Tempesta, David T Bailey, Rebecca L Nichols, F Joseph Daugherty, Delano R Freeberg, “Efficient method for producing compositions enriched in total phenols”, WO 2004047847, 2004. |
| [139] | John A Manthey, “Fractionation of orange peel phenols in ultrafiltered molasses and mass balance studies of their antioxidant levels”, Journal of Agricultural and Food Chemistry, vol.52,no.25, pp.7586-7592, 2004. |
| [140] | Chevalley, I. Antioxidant compositions of vegetal origin and process for their preparation. EP 1541159, 2003. |
| [141] | Peter Lorenz, Ines Roennefahrt, “Concentration and isolation of oregonin from alder barkm useful as antioxidant in cosmetic or therapeutic compositions, includes treating hot water extract with adsorber resin”, DE10247476, 2004. |
| [142] | E M Silva, D R Pompeu, Y Larondelle, H Rogez, "Optimisation of the adsorption of polyphenols from Inga edulis leaves on macroporous resins using an experimental design methodology", Separation and Purification Technology, vol.53,no.3, pp.274-280, 2007. |
| [143] | Judith Kammerer, Dietmar R. Kammerer, Reinhold Carle, “Impact of saccharides and amino acids on the interaction of apple polyphenols with ion exchange and adsorbent resins”, Journal of Food Engineering, vol.98,no.2, pp.230-239, 2011. |
| [144] | Judith Bretag, Dietmar R. Kammerer, Uwe Jensen, Reinhold Carle, “Evaluation of the adsorption behavior of flavonoids and phenolic acids onto a food-grade resin using a D-optimal design”, European Food Research and Technology, vol.228, no.6, pp.985-999, 2009. |
| [145] | Judith Kammerer, Reinhold Carle, Dietmar R Kammerer, “Adsorption and ion exchange: basic principles and their application in food processing”, Journal of Agricultural and Food Chemistry, vol.59,no.1, pp.22-42, 2010. |
| [146] | Andreas Schieber, Petra Hilt, Petra Streker, Hans-Ulrich Endreß, Christine Rentschler, Reinhold Carle, “A new process for the combined recovery of pectin and phenolic compounds from apple pomace”, Innovative Food Science and Emerging Technologies, vol.4,no.1, pp.99-107, 2003. |
| [147] | Andrés Moure, Patricia Gullón, Herminia Domínguez, Juan Carlos Parajó, “Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals”, Process Biochemistry, vol.41, no.9, pp.1913-1923, 2006. |
| [148] | Antonio Lama-Muñoz, Guillermo Rodríguez-Gutiérrez, Fátima Rubio-Senent, Juan Fernández-Bolaños, “Production, characterization and isolation of neutral and pectic oligosaccharides with low molecular weights from olive by-products thermally treated”, Food Hydrocolloids, vol.28,no.1, pp.92-104, 2012. |
| [149] | Georg M Weisz, Lena Schneider, Ute Schweiggert, Dietmar R Kammerer, Reinhold Carle, "Sustainable sunflower processing - I. Development of a process for the adsorptive decolorization of sunflower[Helianthus annuus L.] protein extracts", Innovative Food Science and Emerging Technologies, vol.11,no.4, pp.733-741, 2010. |
| [150] | Sissel Løkra, Reidar Barfod Schüller, Bjørg Egelandsdal, Bjørn Engebretsen, Knut Olav Strætkvern, “Comparison of composition, enzyme activity and selected functional properties of potato proteins isolated from potato juice with two different expanded bed resins”, LWT - Food Science and Technology, vol.42,no.4, pp.906-913, 2009. |
| [151] | Beatriz Gullón, José Luis Alonso, Juan Carlos Parajó, "Ion-exchange processing of fermentation media containing lactic acid and oligomeric saccharides", Industrial and Engineering Chemistry Research, vol.49, no.8, pp.3741-3750, 2010. |
| [152] | C Nobre, M J Santos, A Dominguez, D Torres, O Rocha, A M Peres, I Rocha, E C Ferreira, J A Teixeira, L R Rodrigues, "Comparison of adsorption equilibrium of fructose, glucose and sucrose on potassium gel-type and macroporous sodium ion-exchange resins", Analytica Chimica Acta, vol.654,no.1, pp.71-76, 2009. |
| [153] | J Nowak, K Gedicke, D Antos, W Piatkowski, A Seidel-Morgenstern, "Synergistic effects in competitive adsorption of carbohydrates on an ion-exchange resin", Journal of Chromatography A, vol.1164, no.1-2, pp.224–234, 2007. |
| [154] | Pia Saari, Heikki Heikkilä, Markku Hurme, "Adsorption equilibria of arabinose, fructose, galactose, glucose, mannose, rhamnose, sucrose, and xylose on ion-exchange resins", Journal of Chemical and Engineering Data, vol.55,no.9, pp. 3462-3467, 2010. |
| [155] | Katarína Vaňková, Pavel Ačai, Milan Polakovič, "Modelling of fixed-bed adsorption of mono-, di-, and fructooligosaccharides on a cation-exchange resin", Biochemical Engineering Journal, vol.49,no.1, pp.84-88, 2010. |
| [156] | S Ranil Wickramasinghe, David L. Grzenia, "Adsorptive membranes and resins for acetic acid removal from biomass hydrolysates", Desalination, vol.234, pp.144-151, 2008. |
| [157] | Seoung-Chul Baek, Yun-Joong Kwon, "Optimization of the pretreatment of rice straw hemicellulosic hydrolysate for microbial production of xylitol", Biotechnology and Bioprocess Engineering, vol.12,no.4, pp.404-409, 2007. |
| [158] | Larissa Canilha, Walter Carvalho, Maria das Graças de Almeida Felipe, João Batista de Almeida e Silva, Marco Giulietti, "Ethanol production from sugarcane bagasse hydrolysate using Pichia stipitis", Applied Biochemistry Biotechnology, vol.161,no.1-8, pp.84-92, 2010. |
| [159] | Anuj Kumar Chandel, Rajeev Kumar Kapoor, Ajay Singh, Ramesh Chander Kuhad, "Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501", Bioresource Technology, vol.98,no. 10, pp.1947-1950, 2007. |
| [160] | Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, “Antioxidant activity of liquors from aqueous treatments of Pinus radiata wood”, Wood Science and. Technology, vol.39, no.2 ,pp.129-139, 2005. |
| [161] | Ayako Nishizawa-Yokoi, Yukinori Yabuta, Shigeru Shigeoka, “The contribution of carbohydrates including raffinose family oligosaccharides and sugar alcohols to protection of plant cells from oxidative damage”, Plant Signaling and Behavior, vol.3,no.11, pp. 1016-1018, 2008. |
| [162] | Alistair S. Grandison, Athanasios K. Goulas, Robert A. Rastall, “The use of dead-end and cross-flow nanofiltration to purify prebiotic oligosaccharides from reaction mixtures”, Songklanakarin Journal of Science and Technology, vol.24 (Suppl), pp.915-928, 2002. |
| [163] | Gil Garrote, José Manuel Cruz, Herminia Domínguez, Juan Carlos. Parajó, "Valorisation of waste fractions from autohydrolysis of selected lignocellulosic materials" Journal of Chemical Technology & Biotechnology, vol.78,no.4, pp.392-398, 2003. |
| [164] | Gil Garrote, José Manuel Cruz, Herminia Domínguez, Juan Carlos Parajó, “Non-isothermal autohydrolysis of barley husks: Product distribution and antioxidant activity of ethyl acetate soluble fractions”, Journal of Food Engineering, vol.84,no.4, pp.544-552, 2008. |
| [165] | María Jesús González-Muñoz, Herminia Donínguez, Juan Carlos Parajó, "Depolymerization of xylan-derived products in an enzymatic membrane reactor", Journal of Membrane Science, vol.320,no.1-2, pp.224-231, 2008. |
| [166] | Patricia Gullón, María Jesús González-Muñoz, Herminia Domínguez, Juan Carlos Parajó, "Membrane processing of liquors from Eucalyptus globulus autohydrolysis", Journal of Food Engineering, vol.87,no.2, pp.257-265, 2008. |
| [167] | María Jesús González-Muñoz, Valentín Santos, Juan Carlos Parajó, "Purification of oligosaccharides obtained from Pinus pinaster hemicelluloses by diafiltration", Desalination and Water treatment, vol 27, no.1-3, pp.48-53, 2011. |
| [168] | María Luisa Soto, Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, “Charcoal adsorption of phenolic compounds present in distilled grape pomace”, Journal of Food Engineering, vol.84,no.1, pp.156-163, 2008. |
| [169] | Enma Conde, Andrés Moure, Herminia Domínguez, Juan Carlos Parajó, "Purified phenolics from hydrothermal treatments of biomass: Ability to protect sunflower bulk oil and model food emulsions from oxidation", Journal of Agricultural and Food Chemistry, vol.59,no. ,pp.9158-9165, 2011. |
| [170] | Enma Conde, Mª del Mar Castro López, Andrés Moure, José Manuel López Vilariño, Herminia Domínguez, Mª José Abad López, Victoria González Rodríguez, "An approach to assess the synergistic effect of natural antioxidants on the performance of the polypropylene stabilizing systems", Journal of Applied Polymer Science, vol.126,no.6, pp.1852 - 1858, 2012 |

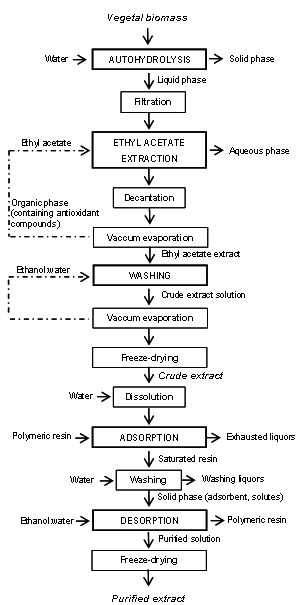
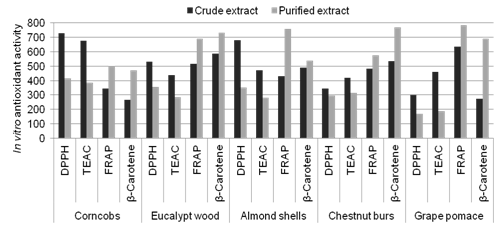
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML