-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(1A): 132-134
doi:10.5923/c.nn.201601.25

Synthesis of Ta2O5 Nanotubes for Photogeneration of H2
Maurício O. Vaz 1, Marco V. Becker 1, Iasmim Biasi 1, Melissa M. Rodrigues 1, 2, Tiago C. Severo 3, 4, Célia de F. Malfatti 2, Sérgio R. Teixeira 1
1Instituto de Física, Universidade Federal do Rio Grande do Sul, (UFRGS), Brazil
2Departamento de Metalurgia, Escola de Engenharia, Universidade Federal do Rio Grande do Sul (UFRGS), Brazil
3Programa de Pós-Graduação em Microeletrônica (PGMICRO), Universidade Federal do Rio Grande do Sul (UFRGS), Brazil
4Centro de Ciências Exatas da Natureza e de Tecnologia, Universidade de Caxias do Sul (UCS-CARVI), Brazil
Correspondence to: Tiago C. Severo , Programa de Pós-Graduação em Microeletrônica (PGMICRO), Universidade Federal do Rio Grande do Sul (UFRGS), Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this work was the optimization of nanotube synthesis parameters maximizing the H2 photoproduction by water splitting. To reach this maximization, the anodization of Ta (Tantalum) was investigated by variation of electrical parameters during process. In this work variations of 1, 5, 10, 25 and 50V/s were applied during 20 minutes / each sample. After the anodizing process, vertical nanotubes were grown with well-defined morphology and aligned with the substrate. All samples were crystallized at 800°C for 1 hour and an orthorhombic form was visualized by Scanning Electron Microscopy. The photocatalytic activity of Ta2O5 nanotubes was evaluated from the photolysis of water, using a quartz reactor and a solar simulator 240W-Xe/Hg lamp. The H2 production was quantified by Gas Chromatography. Focused at the hydrogen production, it was observed that the best sample was obtained with a rate of increase of 1V/s.
Keywords: Ta2O5, Nanotubes, Water Splitting; H2 Production
Cite this paper: Maurício O. Vaz , Marco V. Becker , Iasmim Biasi , Melissa M. Rodrigues , Tiago C. Severo , Célia de F. Malfatti , Sérgio R. Teixeira , Synthesis of Ta2O5 Nanotubes for Photogeneration of H2, Nanoscience and Nanotechnology, Vol. 6 No. 1A, 2016, pp. 132-134. doi: 10.5923/c.nn.201601.25.
Article Outline
1. Introduction
- Presently, the investigation of clean and renewable sources of energy to replace fossil fuel is a significant research field in nanotechnology. [1] In this context, the research of hydrogen (H2), as a fuel, gained significance, because it can generate electricity without emission of polluting gases into atmosphere. A possible reaction for H2 generation is the dissociation of water (H2O) into hydrogen (H2) and oxygen (O2) in the presence of sunlight and a semiconductor metal oxide photocatalyst. This process is known as water splitting [2] and occurs by adjustments of the photocatalyst energy bands to visible light. In this process, the reactions of oxidation (forming O2) and reduction of water (forming H2) occurring on the surface of the photocatalyst, which exhibits the functions of anode and cathode. This is due to the fact that the catalyst is a metal oxide semiconductor, which gives a structure of driving band (BC) separated from the valence band (VB) by a known energy interval per band gap, characteristic for each compound. [3-5]For the reaction to occur, the energy bands must be in proper positions, BC with less potential than the reduction potential of the H+/H2 (0.0EV) and BV with higher potential than the oxidation potential of H2O / O2 (1,23eV). Thus, the band gap of the semiconductor metal oxide used should be at least 1.23 eV. [3-5]For this reason, the Tantalum Pentoxide (Ta2O5) is a promising compound, because it has a band gap of about 4.0 eV, proper positioning of the band energies and excellent chemical stability in aqueous solutions. [3-5] The reaction in the photocatalyst surface is as follows: the valence band electrons are excited by the energy radiated to the conduction band, generating gaps in the valence band. The pairs of electron-hole, when not undergoing recombination, migrate toward the semiconductor surface that respectively reduce and oxidize water molecules.During the reaction, it is used a sacrificial agent, such as ethanol, to increase the efficiency of photocatalytic reaction. For our reactions, we used ethanol as a sacrificial agent, which acts by donating electrons to the valence band and not allowing the recombination of the electrons of the conduction band.In recent years, the study of these photocatalysts has reduced the size of their structures for nanoscale and it was able to improve performance with greater efficiency. [3-5]The production of photocatalyst is made by means of an anodic reaction. The anodizing process is an electrochemical able to create a protective oxide layer on the surface of a metal. During this process two simultaneous reactions occur, the oxidation of the anode and the reduction of the cathode.The electric potential creates an intense electric field between the electrodes and causes the oxygen ions present in the electrolyte migrate to the surface of the anode, leading to growth of tantalum pentoxide. The formation of nanotubes is due to the use of hydrofluoric acid, as F-ions in solution to promote dissolution of the metal oxide, forming nanopores that deepens due to the generated electric field and grow increasingly resulting in the nanotubes. [3-5]The aim of this study was to optimize the nanotube synthesis parameters and to maximize the photoproduction of H2.
2. Methodology
- For the anodizing, it was used an electrolyte solution consisting 95% by volume sulfuric acid (H2SO4), 1% hydrofluoric acid (HF) and 4% of distilled water, in a plastic beaker immersed in an ultrasound thermal bath at the temperature of 40°C.Within the solution are submerged two electrodes, one containing metallic tantalum (anode) and other containing metallic copper (cathode), subjected to electric potential. In addition, a voltage source, voltage source controller, computer and software for controlling the applied voltage ramp and for storing the data of electrical current obtained during the process.The nanotubes of Ta2O5 were formed under different voltage increase rates (1, 5, 10, 25 e 50 V/s) for 20 minutes, analyzed by Scanning Electron Microscopy (SEM). The grain size was evaluated by X-ray diffraction using the Scherrer equation.The photocatalytic activity of Ta2O5 nanotubes was evaluated by hydrogen photogeneration from the photolysis of water, in a double walled quartz reactor with room temperature water circulation (25°C) to avoid overheating. The reactor was exposed to a solar simulator that contained a lamp of Xe/Hg of 240W. The gaseous products of the photocatalytic reaction were quantified by gas chromatography using argon as carrier gas. The generated gas was analyzed at 30-minute intervals through sample collections using a syringe of gas with valve. The reaction of hydrogen photogeneration was analyzed by 150 min.
3. Discussion of Results
- The strategy used in this study was to investigate the influence of increased tension rate regarding the morphology of the Ta2O5 nanotubes; consequently, to check the influence of morphology in the production of H2.
3.1. Effect of Rate of Increase Tension on Ta2O5 NTs Formation
- In this study, it was found that the variation of the strain rate did not influence the formation of NTs of Ta2O5. In Figure 1, you can see the morphology of Ta2O5 NTs formed, rated by scanning electron microscopy (SEM).
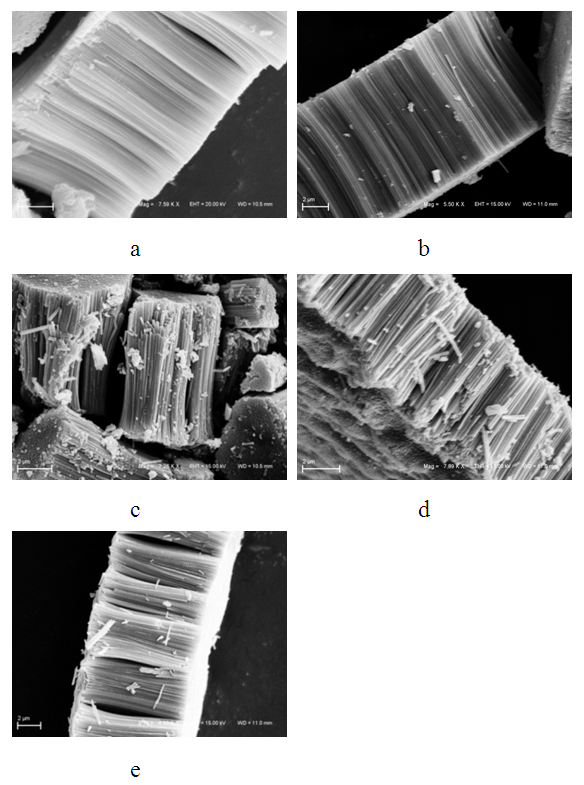 | Figure 1. SEM image of the NTs formed with strain rate: a) 1 v/s; b) 5 v/s; c) 10 V/s; d) 25 v/s e e) 50 v/s at time 20 min |
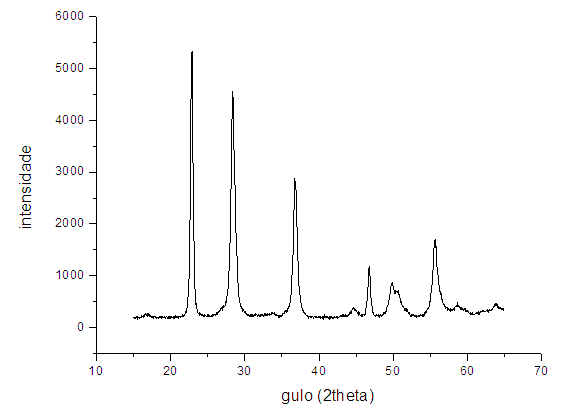 | Figure 2. Diffractogram with features JSPD (25-0922). [6] |
3.2. Hydrogen Production
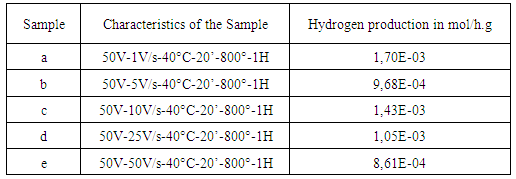
- The photocatalytic activities of the NTs of Ta2O5 obtained by anodizing process, under different strain rates increase, were evaluated by water photolysis process. Table 1 shows the results of obtained hydrogen production of different NTs of Ta2O5 formation.The results shown in Table 1 showed decreasing production of H2 as a function of increasing strain rate.
|
4. Conclusions
- According to the results presented, it was observed that the anodizing of Ta metal resulted in the formation of nanotubes vertically aligned, independent of the voltage rise rate used. Also, it was found that the crystal structure obtained in all NTs was orthorhombic after heating treatment at 800°C for 1 hour. The photocatalytic activity of the NTs of Ta2O5 obtained by anodizing process, under different rates strain increase, was evaluated by water photolysis process and the H2 production in all tested samples was observed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML