-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(1A): 110-112
doi:10.5923/c.nn.201601.21

Nano-crystalline Silicalite-1: Synthesis, Characterization and Application in Biofuel Synthesis
Sunita Barot , Rajib Bandyopadhyay
Pandit Deendayal Petroleum University, School of Technology, Gandhinagar, India
Correspondence to: Sunita Barot , Pandit Deendayal Petroleum University, School of Technology, Gandhinagar, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Transesterification of triacetin was carried out over potassium modified nano-crystalline Silicalite-1. Thenano-Silicalite-1 was synthesized via hydrothermal method and characterized by XRD, SEM, BET and FTIR analysis. Synthesized nano-Silicalite-1 was transformed into efficient heterogeneous base catalyst by impregnating with potassium nitrate. The modified catalyst shows excellent reactivity in transesterification of triacetin. The reaction parameters such as methanol to triacetin molar ratio, reaction time and reaction temperature were also optimized.
Keywords: Nano-Silicalite-1, Triacetin, Transesterification, Biofuel
Cite this paper: Sunita Barot , Rajib Bandyopadhyay , Nano-crystalline Silicalite-1: Synthesis, Characterization and Application in Biofuel Synthesis, Nanoscience and Nanotechnology, Vol. 6 No. 1A, 2016, pp. 110-112. doi: 10.5923/c.nn.201601.21.
1. Introduction
- Transesterification reaction is commercially used for biodiesel synthesis in presence of liquid alkaline hydroxides. But such reaction needs pretreated feedstock (to reduce moisture and FFA) and post processing of products, it ultimately results into the higher production cost. On the other hand heterogeneous catalysts are highly active, selective, reusable and non-corrosive; thereby more advantageous. Number of literature studies reports the use of heterogeneous acid and base catalyst in transesterification reaction. Researchers are devoting the efforts for the development of novel catalyst with high activity as well as stability. For example, Mi Jin Kim et al. studied the transesterification of model triglycerides over rehydrated Mg-Al hydrotalcite, and showed high activity of fully rehydrated hydrotalcite due to generation of Brönsted basic sites and the rapid migration of methanol in the mesopores [1]. Yanhong Zu et al. have prepared series of catalysts by loading CaO over various porous carbon materials, and studied its activity in transesterification of triacetin [2]. Besides these alkali and alkaline earth metal oxides [3], modified zeolites [4], metal doped alumina/silica [5-7], Amberlyst-15 [8], Nafion-SAC-13 and polyaniline sulphates [9], are some of the catalysts that have been reported for transesterification.Nano-crystalline zeolites are more efficient catalyst than the conventional micron sized zeolites due to their enhanced surface area and reduced length of diffusion path. In current studies we have synthesized nano-Silicalite-1 and modified its surface properties by potassium impregnation. The synthesized heterogeneous catalyst when used for transesterification reaction of model triglyceride triacetin was found to be highly efficient giving 98 % conversion with high selectivity towards methyl acetate.
2. Experimental
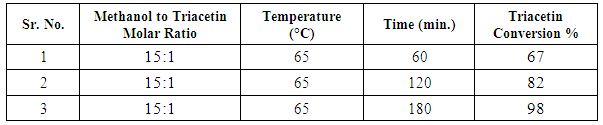
- Nano-Silicalite-1 was synthesized by conventional hydrothermal synthesis; using Tetra Propyl Ammonium Hydroxide (TPAOH) and Tetra Ethyl Ortho Silicate (TEOS) as reaction precursors. Potassium nitrate was used as source of potassium; and loaded on Nano-Silicalite-1 (1 wt % of K per gram of support) by wetness impregnation method. Prior to metal loading and catalytic experiments, as-synthesized nano-Silicalite-1 was subjected to physio-chemical characterization. Crystallinity and phase purity was determined by powder X-ray diffraction using nickel filtered CuKα radiation with wavelength λ=1.5406 Ǻ over PANalytical X’Pert. The crystal size and morphology was evaluated by SEM images taken over Fe-SEM (ZEISS). Nitrogen isotherms were obtained over Micromeritics ASAP 2020 surface area analyzer. Samples were degassed at 200°C for 4 hr prior to analysis. FTIR was recorded over PerkinElmer’ spectrometer model ‘Spectrum Two’ in the range of 400-4000 cm-1.Transesterification of triacetin with methanol was performed in two-neck round-bottom flask attached with reflux condenser over magnetic stirrer. Mixture of triacetin and methanol, with required molar ratio, was charged in round bottom flask and heated at 65°C temperature. After attaining the steady temperature required amount of catalyst was added into the reaction mixture. After completion of reaction, the product mixture was centrifuged to separate the solid catalyst. Product analysis was carried out over Schimadzu gas chromatograph (GC 2025) equipped with Rtx-1 capillary column and FID detector. Identification of product components were done by comparison with authenticate samples and finally by gas chromatograph – mass spectrometry (Agilent GCMS 7890).The effect of various reaction parameters like triacetin / methanol molar ratio, reaction time, and temperature were studied to optimize the reaction conditions for maximum conversion.The effect of triacetin / methanol molar ratio on triacetin conversion was studied, by varying the mole ratio of triacetin and methanol as 1:6, 1:12, 1:15 with 0.3 g catalyst at 60°C for 60 min. The obtained results shows (Figure 4) that triacetin conversion increases with increase in triacetin/ methanol molar ratio as expected and reaches maximum % at 1:12 molar ratio. Further increase in molar ratio, results into very small increase in the conversion, and hence 1:12 is considered as optimum molar ratio for highest conversion.
3. Results and Discussions
- The powder XRD pattern of nano-Silicalite-1 is given in Figure 1, it matches completely with the literature reference samples [10]. Figure 2 shows SEM images of Silicalite-1 with average crystal size of 30-40 nm. Nitrogen isotherm data shows BET surface area 506.36 m2/g with average pore diameter of 76.6 Ǻ. Figure 3 shows FT-IR of nano-Silicalite-1.
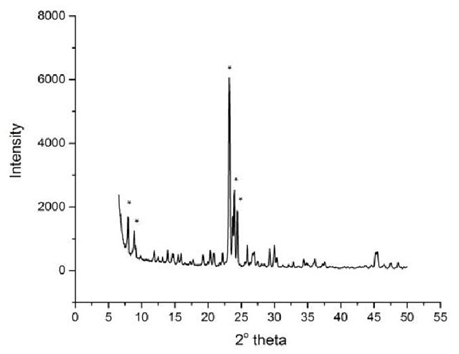 | Figure 1. XRD pattern of as synthesized nano-Silicalite-1 |
 | Figure 2. SEM image of nano-Silicalite-1 |
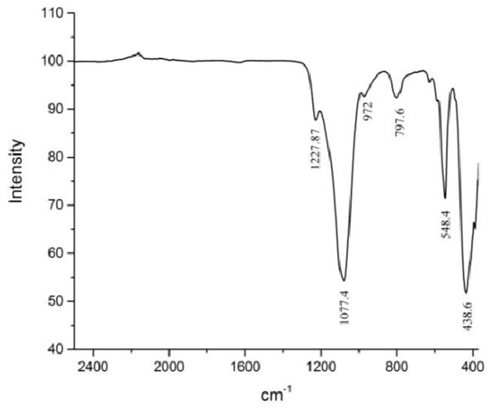 | Figure 3. FT-IR of nano-Silicalite-1 |
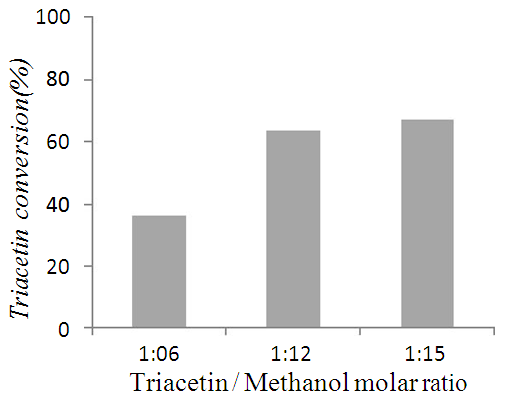 | Figure 4. |
|
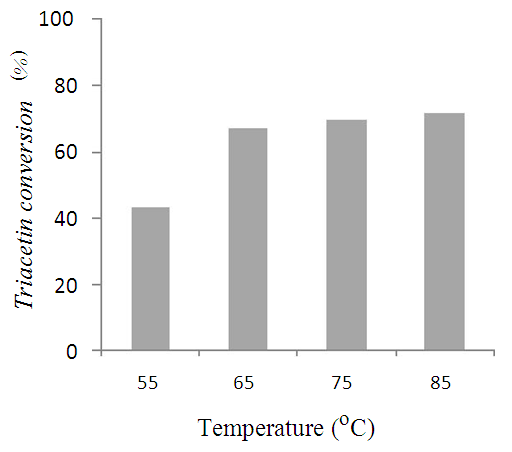 | Figure 5. Effect of reaction temperature on triacetin conversion |
4. Conclusions
- Potassium loaded nano-crystalline Silicalite-1 was synthesized and characterized for physio-chemical properties in current studies. The nano-crystalline catalyst was found to exhibit more surface area and active sites than conventional micron sized catalyst and hence found to be more active. The catalyst was found to be an efficient heterogeneous base catalyst for transesterification reaction of model triglyceride triacetin. The reaction parameters were optimized as 1:12 triacetin/methanol molar ratio, 65°C temperature and 3hr reaction time to obtain high conversion and selectivity for methyl acetate.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML