-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(1A): 88-91
doi:10.5923/c.nn.201601.17

Antibacterial Powders for Medical Application Prepared by Microwave Hydrothermal Assisted Synthesis
Pavel Bazant , Jiri Klofac , Lukas Munster , Ivo Kuritka
Centre of Polymer Systems, Tomas Bata University in Zlin, Zlin, Czech Republic
Correspondence to: Ivo Kuritka , Centre of Polymer Systems, Tomas Bata University in Zlin, Zlin, Czech Republic.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hybrid Ag-ZnO nano-structured microparticles and ZnO hierarchical microparticles were prepared by amicrowave assisted hydrothermal synthesis. Here we present arapid and energy saving one-pot hydrothermal synthesis using amicrowave pressure system. Zinc nitrate and silver nitrate were used as sources of Zn2+ and Ag+. Hexamethylenetetramine (HMTA) was used asa reduction and precipitation agent in water solutions. Crystalline structures of obtained Ag-ZnO and ZnO powders were characterized by an X-ray diffractometer. A scanning electron microscopy was used for the characterization of structure, morphology and particle size of the Ag-ZnO filler. An elemental analysis of the Ag-ZnO filler was made by an Energy dispersive X-ray analysis. The growth mechanism of particles was elucidated. The antibacterial activity was evaluated by an inhibition zone test; one of the materials has shown good antibacterial stability against Staphylococcus aureus, Escherichia coli and Candida albicans.
Keywords: Ag-ZnO, Microwave assisted synthesis, Antibacterial additives
Cite this paper: Pavel Bazant , Jiri Klofac , Lukas Munster , Ivo Kuritka , Antibacterial Powders for Medical Application Prepared by Microwave Hydrothermal Assisted Synthesis, Nanoscience and Nanotechnology, Vol. 6 No. 1A, 2016, pp. 88-91. doi: 10.5923/c.nn.201601.17.
Article Outline
1. Introduction
- The bacterial antibiotic resistance has rapidly increased mainly due to the overuse of antibiotics during the last decade. In addition, treatment of nosocomial infections has become difficult in hospital environments. Among hospital-acquired infections, medical device related infections (MDIs) have been recognized as one of the rapidly growing and significant problems, especially for the insertion of indwelling devices, which come into an intimate contact with the interior of the human body. [1, 2]Nowadays, polymer products in a medical environment are very widely spread. Therefore, the incorporation of inorganic antibacterial agents in to a polymer matrix can be one possible solution for the elimination of nosocomial infections. Inorganic additives combine biocidal and biostatic properties. Therefore, the use of inorganic polymer additives (APAs) compounded into a polymer matrix, so-called antimicrobial polymer systems (APS), can produce a good antibacterial effects for medical application. [3]Metallic Ag and Ag+ ions have a strong antibacterial effect which has been known and used for a long time. Also, metal oxide nanoparticles have attracted quite extensive interest. For instance, zinc oxide (ZnO) representsa biocompatible, biodegradable, and nontoxic ‘‘green’’ material for medical applications and environmental science. ZnO is a semiconductor which also can impart other beneficial properties ofa composite material than metals; nevertheless a considerable antibacterial efficiency is among them. [4]Metal–semiconductorssilver-zinc oxide (Ag-ZnO) were shown to be more efficient than a single component due to synergistic effects of a metal/semiconductor combination. [5-7]Several methods have been reported to prepare Ag-ZnO nano-composites, such as photoreduction method, hybrid induction and laser heating techniques, impregnation photolysis method, ultrasonic spray pyrolysis, flame spray pyrolysis and many others. [8]In this paper, we report our own method for the preparation of antibacterial additives ZnO and Ag-ZnO by a microwave (MW)-assisted synthesis in a pressure mode with a further aim to obtain new antibacterial additives for prospective applications in polymer medical devices.
2. Experimental
2.1. Material
- Silver nitrate AgNO3 (purum, ≥99.5%) and zinc nitrate hexahydrate Zn(NO3)2·6H2O (purum, >99%) were purchased from Penta (Prague, Czech Republic). Hexamethylenetetramine (HMT) C6H12N4 (purum, >99%, Fluka) was purchased from Sigma-Aldrich (Prague, Czech Republic) and subsequently used as a precipitation and reduction agent and a growth modifier. Demineralised water was used in these experiments.
2.2. Synthesis of ZnO and Ag-ZnO
- In the first case, the solution of 0.1 M Zn(NO3)2·6H2O was mixed together with 0.1 M C6H12N4 and ZnO was prepared. In the second case, 0.01 M AgNO3was further added and Ag-ZnO was formed. The total amount of used water was 60 mL. For the preparation of the materials a commercial pressure MW system Mars 5 (CEM Corporation) was used. Identical solutions were prepared in smaller amounts, as they needed to be mixed prior synthesis and filled in the Teflon reaction vessel (XP-1500 Plus), sealed and heated in the microwave oven. The reaction solution in the vessel was irradiated by MW at moderate power of 400 W to ensure a temperature regulation. The solution was heated to 120°C for 30 minutes. When the reaction was completed, the vessel was left to cool down at the room temperature and then unsealed. Obtained suspensions were filtered, washed with demineralised water and finally collected powders were dried in the air at 37°C for overnight.
2.3. Characterization
- Powder X-ray diffraction analysis for crystal phase identification was performed on a PANalyticalX´Pert PRO X-ray diffractometer (PANalytical, The Netherlands) in the diffraction 2θ angle range 25-85°, using Cu Kα1 radiation (λ = 1.5418 Å). The crystallite size D of the two samples were estimated using the Scherrer’s equation, (0.9λ)/ (β cosθ), by measuring the line broadening (β, the full width at half-maximum, corrected by the instrument response) of the main intensity peak for zinc oxide (101) and (111) for silver, where λ is the wavelength of Cu Kα radiation, and 2θ is the Bragg’s angle.The morphology of samples was observed by the scanning electron microscope (SEM) Phenom Pro (Phenom-World B.V., The Netherlands). The elemental microanalysis has been carried out with the use of a scanning electron microscope Nova NanoSEM (FEI, The Czech Republic) by the energy dispersive X-ray analyser TEAM EDS Analysis System integrated into the SEM.
2.4. Testing Antibacterial Activity
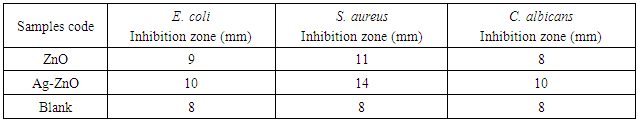
- The antibacterial testing was performed against Staphylococcus aureus CCM 4516, Escherichia coli CCM 4517 and Candida albicans CCM 8215. The amount of 0.1g of obtained ZnO and Ag-ZnO powders was dispersed in 1 mL of demineralised water and homogenised in ultrasonic bath for 15 minutes. Then, 10 μL of each suspension was applied on filter paper with the diameter 8 mm. Prepared specimens were dried at the room temperature. Specimens (diameter 8 mm) were put into nutrient agar which was inoculated with bacteria (approximately 108 colony forming unit (CFU) per millilitre). After 24 h incubation at 37°C, the diameters of the inhibition zones which appeared on the surface were measured in five directions. Subsequently, average values were used for the calculation of the inhibition zone area. This gives the measure of the antibacterial activity of the studied samples. Each test was conducted with six specimens. Blank samples without a suspension were prepared and tested in the same procedure.
3. Results and Discussion
3.1. Morphology and Structure of Prepared ZnO and Ag-ZnO Particles
- SEM microphotographs are shown in Figure 1a and 1b. The images were taken by the BSE detector that allows the distinguishing of particles composition by material (greyscale) contrast showing heavier elements brighter. The microparticles of sample ZnO that can be seen in the Figure 1a have geometrically regular hexagonal shapes assembled into star-like or flower-like particles, where the constituting rods resemble flower petals rather than hexagonal prisms. The length of all observed rods or petals is up to 2 µm. On the top of ZnO rods, it is possible to see small cap buttons. The diameter of these buttons is up to 0.5 µm.
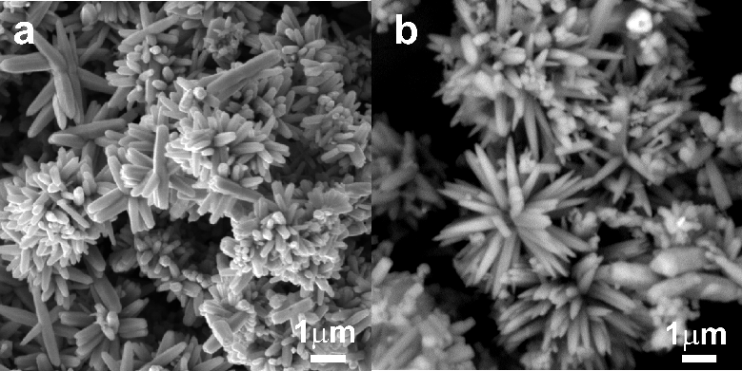 | Figure 1. SEM pictures of (a) ZnO and (b) Ag-ZnO particles |
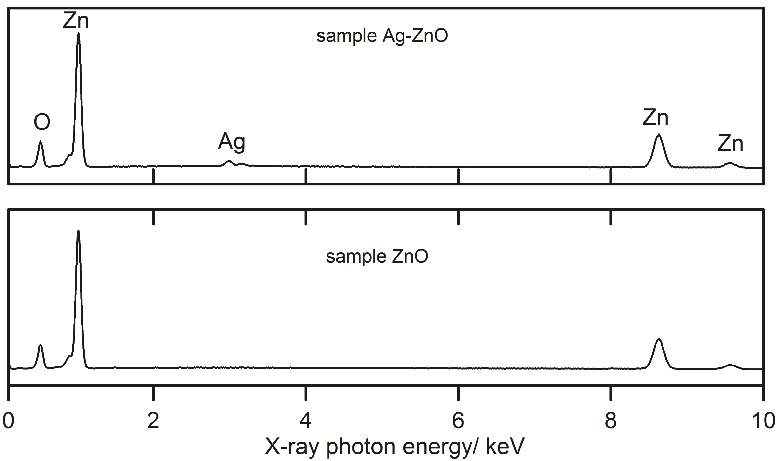 | Figure 2. EDX spectra of sample Ag-ZnO and ZnO |
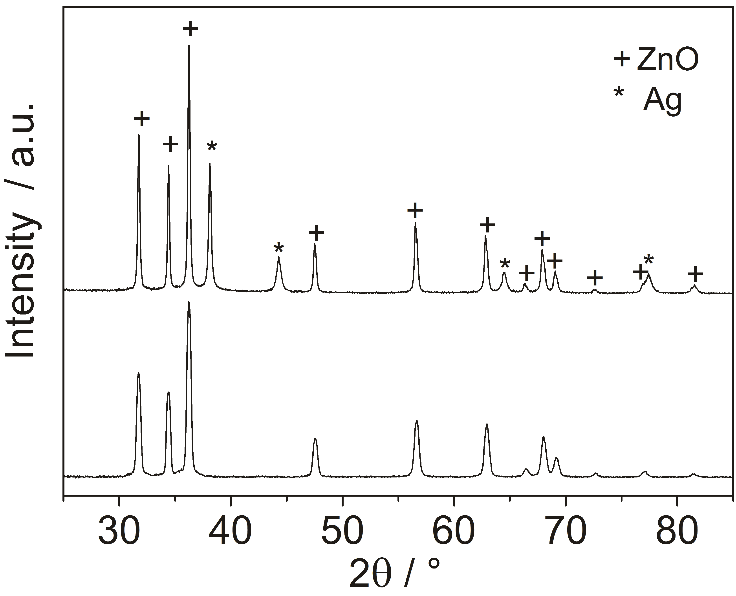 | Figure 3. XRD patterns of the ZnO and Ag-ZnO particles |
3.2. Antibacterial Activity of ZnO and Ag-ZnO Additives
- The antibacterial activity of ZnO and Ag-ZnO and nanoparticles was tested by the agar disk diffusion test and results are shown in Table 1. The presence of an inhibition zone clearly indicated the antibacterial effect of both, ZnO and Ag-ZnO nanoparticles.
|
4. Conclusions
- Ag-ZnO nano-structured microparticles and ZnO hierarchical microparticles were synthesized by the microwave assisted hydrothermal synthesis in the pressure system. The obtained powder materials consist of metallic nanosilver and semiconducting ZnO microparticles with a multiscale morphology. The Antibacterial additive Ag-ZnO has shown good antibacterial activity against tested bacteria, especially against S. aureus. This fact testifies that antibacterial polymer systems containing fillers such as Ag-ZnO nanostructured microparticles have potential to be used as additives for plastic medical devices as well as in other industries requiring an antibacterial action of the material, for example sanitary, hygienic or other interior applications.
ACKNOWLEDGEMENTS
- This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic – Program NPU I (LO1504).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML