-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(1A): 65-67
doi:10.5923/c.nn.201601.12

TiO2 Nanotubes Decorated with Carbon Nanoparticles for Indigo Carmine Photodegradation
Iasmim Biasi 1, Janine H. Dias 1, Melissa M. Rodrigues 1, 2, Hemerson P. S. Castro 1, Tiago C. Severo 3, Daniel Weibel 4, Célia de F. Malfatti 2, Ricardo R. B. Correia 1, Sérgio R. Teixeira 1
1Universidade Federal do Rio Grande do Sul (UFRGS), Programa de Pós-Graduação em Microeletrônica (PGMICRO), Brazil
2Universidade Federal do Rio Grande do Sul (UFRGS), LAFOS, Instituto de Química, Brazil
3Universidade de Caxias do Sul (UCS-CARVI), Centro de Ciências Exatas da Natureza e de Tecnologia, Brazil
4Universidade Federal do Rio Grande do Sul (UFRGS), LAPEC, Departamento de Eng. Metalúrgica, Brazil
Correspondence to: Tiago C. Severo , Universidade de Caxias do Sul (UCS-CARVI), Centro de Ciências Exatas da Natureza e de Tecnologia, Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this work we investigate the influence of Carbon nanoparticles on the photodegradation of Indigo Carmine dye (ICD) using TiO2 nanotubes (TiO2NTs) decorated with Carbon nanoparticles as photocatalytic. For comparison we have evaluated the ICD photodegradation using pure TiO2 as photocatalyst material. The Carbon nanoparticles have been synthetized by laser ablation of graphite in ethanol and freestanding TiO2 nanotubes by anodization. The systems were characterized by TEM, SEM, FTIR, XRD and Uv-Vis light absorption. The CNPs presented an average diameter around 3nm with a remarkable broadband fluorescence under UV excitation. The TiO2NTs have a diameter average about 100 nm and length around 4 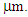
Keywords: Nanoparticles, Titanium dioxide, Indigo carmine, Solar photodegradation, Heterogeneous photocatalysis
Cite this paper: Iasmim Biasi , Janine H. Dias , Melissa M. Rodrigues , Hemerson P. S. Castro , Tiago C. Severo , Daniel Weibel , Célia de F. Malfatti , Ricardo R. B. Correia , Sérgio R. Teixeira , TiO2 Nanotubes Decorated with Carbon Nanoparticles for Indigo Carmine Photodegradation, Nanoscience and Nanotechnology, Vol. 6 No. 1A, 2016, pp. 65-67. doi: 10.5923/c.nn.201601.12.
Article Outline
1. Introduction
- Indigo Carmine dye haspresented serious social and environmental problems due to pollution of the environment by industrial effluents. The waste produced often contains toxic pollutants and it is resistant to conventional treatment systems (coagulation/flocculation, adsorption on activated carbon, precipitation, biological degradation among others). [1] [2] The textile industry, in particular, has high demand for water in its processes, generating large amounts of wastewater, which, generally contains high loads of dissolved salts, surfactants, suspended solids, and organic matter, mainly in the form of complex dye molecules. [3-5] Due to its complex chemical structure, when discarded into the environment without any treatment, the dyes are not easily biodegraded by bacteria present in the system, remaining a long period in the ecosystem. [6] The Indigo Carmine dye is highly toxic and may cause skin and cornea irritations. It has carcinogenic properties that interfere with neural development; and their consumption can be fatal. [7] Fig. 1 shows the two structures in the dyes, noting the NaSO3 - Indigo Carmine group, which confers solubility in aqueous environment. Fig. 1 also shows the chromophore group, responsible for the dying of the color. This consists of a conjugated system with a substituted C = C bond by two groups of the electron donor (NH)and two groups of electron acceptors (C = O). [8]
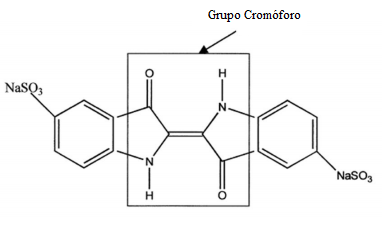 | Figure 1. Molecular structure of the dye indigo carmine |
2. Methodology
- To produce the fluorescent CNPs (carbon nanoparticles)in ethanol by laser ablation method; we used a pulsed Nd-YAG laser set at 1064 nm, operating at 20 Hz with energy about 100 mJ per pulse irradiating no focusing on solutions composed by expanded graphite diluted in ethanol under ultrasonic agitation during two hours. Lastly, this solution was centrifuged in regime of 6000 rpm during 40 min to separate the nanoparticles produced and the residual particles from the initial solution, similar a method developedin [11]. The TiO2NTs were produced by anodization. The anodization process is an electrolytic reaction that consists in growing an oxide layer on a metallic substrate, with addiction of Fluor ions at the electrolyte composition it is possible to create some porous structures as well as nanotubular geometries. As the electrolyte, we used a solution composed by 0.5% wt NH4F (ammonium fluoride), 10% wt distilled water and ethylene glycol. The electrodes were a titanium plate (99.98%) purchased from Sigma Aldrish and cooper as a counter electrode. The syntheses of the nanotubes were performed at room temperature in ultrasonic bath without voltage slope during 1h, after this, the thermal treatment was applied. Lastly, TiO2NTs (8 mg) where added to CNPs solution (2 mL) with stirring for 10 min and dried in a vacuum oven at 80ºC for 12h.The tests of photodegradation of Indigo Carmine were conducted using a 30 ml quartz reactor. The reactor is exposed to a solar simulator that comprisesa lamp of the Xe/Hg of the 240W. The photocatalytic activity of the photocatalysts prepared was evaluated by the percentage of disappearance of Indigo Carmine via UV-Vis, based on the absorption band at 611 nm.
3. Characterization and Discussion of Results
- The transmission electronic microscopy TEM and high resolution HRTEM was employed to observe the morphology and crystalline structure of the produced nanoparticles. Those analyses reveal that the CNPs have a size distribution around 3 nm diameters and the crystalline structure of the C-dots with the distances of the patterns in agreement with the (002) plane of the carbon graphite structure. To understand the nature of bonds and to analyze the composition of the CNPs surface, we measured the FTIR spectra for the solvent and the colloids. Under UV excitation the C-Dots displayed a great fluorescence. To measure the fluorescence spectra was employed the following setup: a pulsed (8 ns) Nd-YAG Laser set at 355 nm irradiated the samples in a quartz cuvette, the fluorescence signal was detected by a SM-240 Spectrometer coupled with an optical fiber perpendicular to excitation beam. By XRD analysis, we observed that TiO2NTs were in anatase phase. With the CNPs attached on the TiO2NTs surface we have performed the Uv-Vis light absorption spectra and compared to the pure TiO2, presenting a yellow-brown coloration due to the CNPs absorptionon UV and IR regions.The result of photobleaching Indigo Carmine proved to be excellent. In just one hour of irradiation, the photodegradation of the Indigo Carmine, using the TiO2NTs/NPCs as catalysts, was complete. The photodegradation percentage of Indigo Carmine obtained was 100%. When used only the TIO2NTs as catalyst, the result of photodegradation of the dye was 95% in only 1-hour exposure to the solar simulator.
4. Conclusions
- According to the results presented, it was observed that the developed photocatalysts were excellent when used on the photodegradation of Indigo Carmine. Also, it was found that the addition of CNPs in the TiO2NTs improves the fotodegradation efficiency.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML