-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(1A): 62-64
doi:10.5923/c.nn.201601.11

HYSYCVD Synthesis of 1D Nanostructures of TiO2
A. L. Leal-Cruz 1, 2, A. Pérez-Aguirre 3, R. Meléndrez-Amavizca 2, A. Vera-Marquina 2, M. Barboza-Flores 2, M. I. Pech-Canul 4, J. Hernandez-Torres 5, J. G. Dominguez-Chavez 6, J. Martínez-Castillo 5
1Departamento de Ciencias Químico Biológicas, Universidad de Sonora, Hermosillo, México
2Departamento de Investigación en Física, Universidad de Sonora, Hermosillo, México
3Departamento de Física, Universidad de Sonora, Hermosillo, México
4Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Unidad Saltillo, Ramos Arizpe, México
5Centro de Investigación en Micro y Nanotecnología, Universidad Veracruzana, Boca del Río, México
6Facultad de Bioanálisis, Universidad Veracruzana, Veracruz, México
Correspondence to: A. L. Leal-Cruz , Departamento de Ciencias Químico Biológicas, Universidad de Sonora, Hermosillo, México.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this work, a synthesis route for 1D nanostructures (nanoribbons and nanofibers) of titanium oxide via hybrid precursor systems chemical vapor deposition (HYSYCVD) is proposed. The route is based on the thermal decomposition of K2TiF6 (solid precursor) in controlled air atmosphere. Synthesized TiO2 corresponds to 1D crystalline nanostructures of Anatase, which can find potential applications in the development of thin-film transistor, dye-sensitized solar cells, and dosimeters. The versatility for synthesizing 1D TiO2 nanostructures by HYSYCVD suggests the potential of the proposed route as economic and large-scale production technique.
Keywords: Synthesis, Nanoribbons, TiO2
Cite this paper: A. L. Leal-Cruz , A. Pérez-Aguirre , R. Meléndrez-Amavizca , A. Vera-Marquina , M. Barboza-Flores , M. I. Pech-Canul , J. Hernandez-Torres , J. G. Dominguez-Chavez , J. Martínez-Castillo , HYSYCVD Synthesis of 1D Nanostructures of TiO2, Nanoscience and Nanotechnology, Vol. 6 No. 1A, 2016, pp. 62-64. doi: 10.5923/c.nn.201601.11.
Article Outline
1. Introduction
- Titanium oxide (TiO2) is a versatile metallic oxide semiconductor (n-type and 3.2 eV band gap) with a wide range of applications in different technological fields ranging from photocatalyst, chemical gas sensor, self-cleaning coating, antiseptic coatings, spintronic devices, dye-sensitized solar cells (DSSCs), thin-film transistor (TFTs) to dosimeters [1, 2]. Interest in ceramics based on nanostructured metallic oxides, such as, 1D nanostructures of TiO2 has increased considerably in recent years, owing to their excellent properties and potential applications in TFTs, DSSCs and dosimeters. Nanostructured TiO2 materials exhibit superior electrical and optical behaviour than the corresponding TiO2 bulk materials. In addition, TiO2 displays superior structural and functional properties in comparison with other oxides, and it is considered an efficient material for electronic devices [1, 3]. Crystalline TiO2 presents polymorphisms, occurring in three polymorphic phases: Rutile, Anatase, and Brookite. The processing conditions and synthesis techniques influence the resulting crystalline structure, the physical and chemical properties. TiO2 can be obtained by different techniques, such as: chemical solution decomposition, two-step wet chemical method, solgel, ultrasonic irradiation, solvothermal method, hydrothermal route, and chemical vapor deposition (gas precursor systems) [2]. An alternative synthesis method for TiO2 is the thermal decomposition of solid precursors or hybrid precursor systems chemical vapor deposition (HYSYCVD). Originally, HYSYCVD was developed by Leal-Cruz and Pech-Canul in 2002 [4, 5], It is a modified CVD method based on thermal decomposition of solid precursors under controlled atmosphere for ceramics synthesis, such as Si3N4, Si2N2O, SiO2, AlF3, and Al2O3 [6]. In this work, a synthesis route via HYSYCVD in the K2TiF6 -air system is proposed with the aim of synthesizing 1D nanostructures of TiO2 intended for TFTs, DSSCs and dosimeters.
2. Experimental Procedure
- TiO2 was synthesized via thermal decomposition of solid precursor under controlled air atmosphere in a HYSYCVD reactor [4, 5]. K2TiF6 was used as titanium solid precursor and decomposed under the following processing conditions: temperature of 923.15 K, processing time of 2.5 h, heating rate of 10 °K/min, and atmospheric pressure. Prior to TiO2 synthesis, K2TiF6 powders were compacted in a steel die (5 mm of diameter and 1mm of height) by biaxial compaction to obtain preforms. Processed preforms were removed and prepared for their characterization by X-ray diffraction (XRD), field emission scanning electron microscopy, and energy dispersive X-ray spectroscopy (FESEM/EDS). Additional, thermoluminescence (TL) characterizations were performed in the obtained materials.
3. Results and Discussion
3.1. Phase Identification by XRD
- Figure 1 shows the XRD patterns corresponding to a representative sample processed by HYSYCVD showing phases associated with the formation of TiO2.Characterization by XRD of processed samples (Figure 1b) reveals the presence of Anatase polymorph of TiO2 (JCPDS No. 00-041-1049; titanium oxide (TiO2) or Anatase; tetragonal, a=b=4.14 Å, c=9.579 Å; and 25.209, 47.874, 37.327, 53.533). K2TiF6 (JCPDS No. 00-073-2110; potassium titanium fluoride (K2TiF6); hexagonal, a=b=5.715 Å, c=4.656 Å; and 41.282, 26.258, 31.728, 17.907) and K2.67TiO0.67F5.33 (JCPDS No. 00-039-0781; potassium titanium oxide fluoride (K2TiOF5); cubic, a=b=c=8.624 Å; and 29.245, 41.845, 17.705, 20.512) are associated with titanium solid precursor and intermediate phases formed during the synthesis of TiO2. TiO2 and K2.67TiO0.67F5.33 (intermediated product) allows confirming the mechanism of TiO2 formation through direct and intermediate routes during decomposition of solid precursor (K2TiF6) predicted and proposed in a previous work [6].
3.2. Morphological Analysis of Phases by FESEM Technique
- Figure 2 shows a representative FESEM image and TL glow curve induced by gamma radiation corresponding to samples processed by HYSYCVD.FESEM results reveal that TiO2 corresponds to nanoribbons and nanofibers. EDS analyses confirm the presence of titanium (T) and oxygen (O) corresponding to chemical elements belonging to TiO2 phase in 1D nanostructures. According to the literature [2, 7], nanoribbons and nanofibers of TiO2 semiconductor can find potential applications in the development of thin-film transistor and dye-sensitized solar cells. On the other hand, the versatility for synthesizing 1D nanostructure of TiO2 by HYSYCVD at relatively low temperature, short times, and in situ process suggests the potential of the proposed route for the economic and large-scale production of 1D nanostructures of TiO2. Additional characterizations by thermoluminescence (TL) shows that synthesized TiO2 presents luminescent properties, which makes it promising for potential applications in dosimeters.
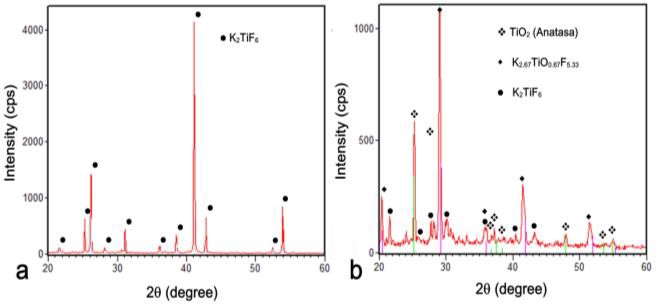 | Figure 1. XRD patterns of K2TiF6 solid precursor before (a) and after (b) processing by HYSYCVD |
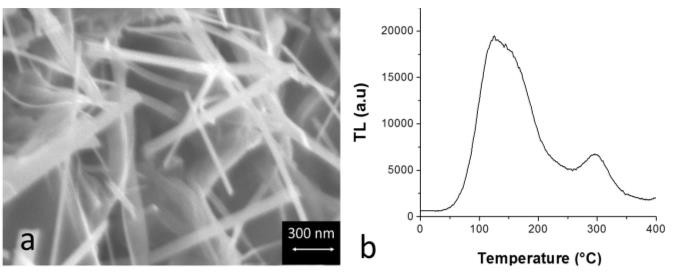 | Figure 2. FESEM photomicrograph (a) and TL glow curve of samples processed via HYSYCVD |
4. Conclusions
- TiO2-nanoribbons were successfully synthesized via the hybrid precursor systems chemical vapor deposition (HYSYCVD) by the thermal decomposition of K2TiF6 under controlled air atmosphere. 1D nanostructures of TiO2 can find potential applications on TFTs, DSSCs and dosimetry. Lastly, HYSYCVD technique shows versatility for synthesizing TiO2 and offers great potential to be considered as economic and large-scale production technique.
ACKNOWLEDGEMENTS
- Authors gratefully acknowledge the Consejo Nacional de Ciencia y Tecnología (CONACYT) of México for financial support under project contract CONACYT 12205-1416009 (Researcher retention program).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML