-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(1A): 25-28
doi:10.5923/c.nn.201601.04

Effect of Thermal Calcinations on the Charge Transfer Resistance of Tantalum Nitride Nanotubes
Sherdil Khan 1, Tiago Cassol Severo 2, 3, Marcos Jose Leite Santos 4, Sérgio Ribeiro Teixeira 1, 3
1Instituto de Fisica, Universidade Federal do Rio Grande do Sul, Av. Bento Goncalves, POA-RS, Brazil
2Centro de Ciências Exatas da Natureza e de Universidade de Caxias do Sul (UCS-CARVI), Bento Gonçalves, RS, Brazil
3Universidade Federal do Rio Grande do Sul (UFRGS), L3FNano, Programa de Pós-Graduação em Microeletrônica (PGMICRO)
4Instituto de Quimica, Universidade Federal do Rio Grande do Sul, POA-RS, Brazil
Correspondence to: Tiago Cassol Severo , Centro de Ciências Exatas da Natureza e de Universidade de Caxias do Sul (UCS-CARVI), Bento Gonçalves, RS, Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Nanotubes of Ta3N5 were synthesized by thermal Nitridation of Ta2O5 nanotubes under ammonia gas environment. The effect of Nitridation temperature and time was evaluated. Electrochemical impedance spectroscopy was applied to study the charge transfer resistance across the Ta3N5 NTs/electrolyte interface. It was observed, at higher temperatures tubular morphology can be preserved for short nitridation time. The electrochemical impedance spectroscopy (EIS) revealed that nitridation conditions such as temperature and time strongly influence the interfacial charge transportation; affecting the photoelectrochemical (PEC) activities of the photoanodes.
Keywords: Photoelectrolysis, Ta3N5 nanotubes, Photoanode
Cite this paper: Sherdil Khan , Tiago Cassol Severo , Marcos Jose Leite Santos , Sérgio Ribeiro Teixeira , Effect of Thermal Calcinations on the Charge Transfer Resistance of Tantalum Nitride Nanotubes, Nanoscience and Nanotechnology, Vol. 6 No. 1A, 2016, pp. 25-28. doi: 10.5923/c.nn.201601.04.
1. Introduction
- In recent years, tantalum nitride (Ta3N5) has received an increasing attention as a photoanode for hydrogen production via photoelectrochemical water splitting under visible light irradiation. [1, 2] Its band structure is near optimal for over all water splitting as it fulfils the straddling of water redox potentials in the band gap. [3] The use of Ta3N5 for hydrogen production via water splitting is relatively new compared to other semiconductors such as Ta2O5, hematite, TiO2 and further investigations are required to better understand its physicochemical properties and to improve its photocatalytic performance. [4-6] Nanotubes of Ta3N5 are of great potential to improve the photoelectrochemical performance of the electrode mainly due to large active surface area. [7, 8] To synthesize Ta3N5 nanotubes at higher temperatures the precursor nanotubes should be synthesized in a manner that helps increasing their adherence on the substrate along with increasing wall thickness. [2] The electrochemical impedance spectroscopy is an attractive tool to investigate the semiconductor- electrolyte interfaces. [5] The interpretation of experimental results can be modelize by equivalent circuits analogy that helps to understand the electrochemical systems provided that particular arrangement of circuit elements in a network possesses physical meaning. [9, 10] Therefore, utilizing electrochemical impedance spectroscopy and equivalent circuits analogy to study the interfacial charge transportation properties of Ta3N5 NTs synthesized under different thermal calcinations conditions are interesting to optimize the photoanode for improved photoelectrochemical activity.In this work, a detailed investigation on the effect of a systematic thermal calcination on the interfacial charge transportation resistance for Ta3N5 nanotubular photoanode is presented.Ta3N5 nanotubes were prepared starting from Ta2O5 nanotubes obtained by anodization. The anodization was carried out in a homemade two electrode sono-electrochemical setup using a Cu disk as cathode and Ta sheet (Alfa Aesar, purity 99.95%) as anode. The anodization electrolyte was a mixed solution of H2SO4 (Lab-Synth Products Laboratory LTD, 98.0%) + 1 vol% of HF and 4 vol% of distilled water. A DC voltage of 50V was applied for 20 min by an initial ramping of 10V/s at 10°C of the electrolyte temperature. To synthesize Ta3N5 as-anodized Ta2O5 nanotubes were placed on an alumina boat and inserted into a horizontal quartz tube oven. The heating and cooling rate of the oven was kept at 5°C/min under a constant 100ml/min gas flux consisting of a special mixture of ammonia: argon (1: 9 vol/vol).For comparison three sets of samples have been synthesized: (a) Constant temperature nitridations at 800°C for 1, 2, 3, 5 and 10 hours.(b) Nitridation at 650°C, 700°C, 750°C, 800°C and 900°C for long time of 10 hours.(c) Nitridation at 800°C, 850°C, 900°C and 1000°C for shorter time of 3 hours.For simplicity the samples were labelled as T°C-time, e.g. the sample nitrided at 650°C during 10h was labelled as 650°C-10h.X-ray diffraction (XRD) patterns from the Ta3N5 nanotubes were obtained by using Siemens, D500 diffractometer with Cu Kα radiation (λ = 1.54 Å) at a 2θ range from 10° to 70° with a 0.05° step size and measuring time of 5 s per step. Rietveld refinements were performed using the same methodology as presented in our previous report. [1] [2] Scanning electron microscopy (SEM) was performed using EVO 50 from Zeiss operating at 10 kV. Electrochemical impedance spectroscopy measurements were performed using an Auto-lab (AUT 84503) potentiostat. The experiments were performed in a quartz cell using standard three-electrode setup employing Ta3N5 nanotubes as working electrode, Pt wobbling as counter electrode and Ag/AgCl as a reference electrode. The electrolyte was 0.1 M K4 [Fe (CN)6 (Merck) and 0.1 mM K3 [Fe (CN)6] (Merck) at pH 7.5. Prior to each measurement the electrolyte was purged by argon gas and photoanodes were cleaned by successive cyclic voltammetry scans. The impedance spectroscopy measurements were performed at a frequency range of 100 kHz – 100 MHz with amplitude of 5 mV applying 0.2 V vs Ag/AgCl.
2. Results and Discussion
- Figure 1 displays the morphology of the sample prepared at 800°C for 5 h. It can be seen that the morphology of the nanotubes is sustained due to optimized anodization process used for preparing the starting material i.e. Ta2O5 nanotubes. [2] The as anodized Ta2O5 nanotubes were grown to a length of approximately 1.60 ± 0.03 μm with an average outer diameter and wall thickness of 130 ± 4 nm and 34 ± 2 nm, respectively. [2] However, after the calcinations under ammonia environment the dimensions of the nanotubes decreased. In addition, the grey coloured Ta2O5 sample turned red indicating the phase transition to Ta3N5 nanotubes. Furthermore, the walls of the nanotubes are rough. The features on the walls of the NTs and changes in the geometrical dimensions are related to the density difference between the starting material, Ta2O5 NTs, and the final material, Ta3N5 NTs. [11, 12]
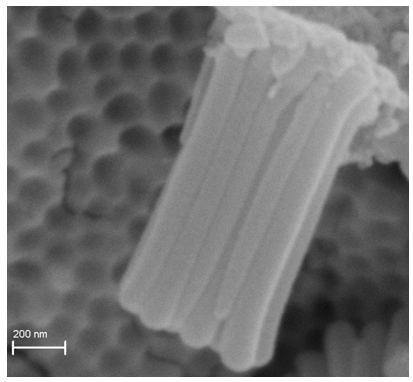 | Figure 1. Ta3N5 nanotubes obtained at 800°C for 5 hours |
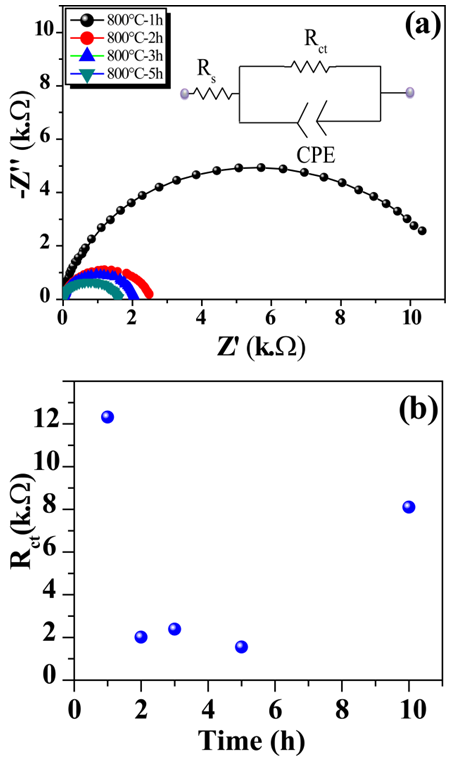 | Figure 2. Nyquist plots of Ta3N5 NTs prepared at different thermal calcinations conditions |
3. Conclusions
- Ta3N5 nanotubes were prepared at different conditions of thermal calcinations under ammonia environment. It was observed that thermal calcination as a function of temperature and time influence the crystalline structure of Ta3N5 nanotubes. The charge transfer resistance across the Ta3N5 nanotubes/electrolyte interface strongly depends on the calcinations conditions such as temperature and time which is related to the crystalline structure obtained at these conditions. It was observed that long time thermal treatments to produce Ta3N5 nanotubes present structural as well as morphological collapse resulting in to highest charge transfer resistance across the interface. Therefore, Ta3N5 nanotubes should be prepared at higher temperature but short times.
ACKNOWLEDGEMENTS
- The authors are grateful to Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Brazil, for the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML