-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(3C): 169-174
doi:10.5923/c.materials.201502.33
Innovative Control Measures of Water Pollution - A Study on Green Chemistry
Motilal Lakavat , L. Nageswara Rao
Caledonian College of Engineering-A University College, CPO Seeb 111, Muscat, Sultanate of Oman
Correspondence to: Motilal Lakavat , Caledonian College of Engineering-A University College, CPO Seeb 111, Muscat, Sultanate of Oman.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
To prevent the concentration of water pollution, efficient safety measures have be employed that prevent point-source and nonpoint-source pollution. Green chemistry solutions should be employed, where it is possible. An effective way to deal with the water pollution problem is to use the least harmful ways to prohibit contaminants. Chromatography methods and separation chemistry principles offer a variety of encouraging methods to attain these attainable goals. Proper remediation has been provided to aid correct contamination of groundwater, which affects nearly hundred million people worldwide. A variability of novel methods has been included for prevention and remediation of water pollution that occasion radical reaction and the utilization of advanced nano-based techniques. This current paper is mainly focusing on current aspect of water pollution control strategies with green chemistry.
Keywords: Green chemistry, Water pollution, Pollution control, Chromatographic methods
Cite this paper: Motilal Lakavat , L. Nageswara Rao , Innovative Control Measures of Water Pollution - A Study on Green Chemistry, American Journal of Materials Science, Vol. 5 No. 3C, 2015, pp. 169-174. doi: 10.5923/c.materials.201502.33.
Article Outline
1. Introduction
- Green chemistry focuses on the reduction, recycling, and/or removal of the use of toxic and hazardous chemicals in production processes by finding creative, alternative routes for making the desired products that reduce the impact on the environment. Green chemistry is a more eco-friendly green alternative to conventional chemistry practices. The green chemistry movement is part of a larger movement ultimately leading to a green economy- namely sustainable development, sustainable business and sustainable living practices [1-3]. Green chemistry can contribute to achieving sustainability in three key areas. First, renewable energy technologies will be the central pillar of a sustainable high-technology civilization. Second, the reagents used by the chemical industry. Third, polluting technologies must be replaced by benign alternatives. The aim of the article is to acquaint the academicians, researchers, scientists and engineers with values and positive impact of green Chemistry in innovation, application and Technology [4].Green chemistry is the design, development, and execution of chemical products and processes to decrease or eliminate the use of substances hazardous to human health and the environment. Members of this relatively new field have been making path breaking efforts to reduce the evil consequences of the chemical industry. Their efforts are also to allow economic progress and environmental growth to proceed in harmony. This new branch of science that includes modification of engineering practices and bioremediation, also promotes catalytic processes and eco-friendly reaction media, as well as the concept of atom economy leading to almost zero waste. As a result, there have been efforts to attain environmentally kind synthesis and various acts have been passed to control and care for pollution, in an attempt to encourage industries and academics to devise novel technologies, processes and educational materials, discouraging the formation or use of hazardous substances. This revolution is rather recent and started in the real intellect in the 1990s, especially in the developed nations like the US, Germany and UK, for instance. Eventually, it is appreciated that while it is necessary to announce enactments and legislations, what is possibly more important is the awareness of detriments not only by the chemists/technologists, the academia and policy makers but also by the common mass in good proportion, to enable generate sense of resistance[5-6].The drinking water originates from various sources such as rivers, lakes, wells, and natural springs. Unfortunately, the originated sources can be effortlessly polluted by point and nonpoint source pollutants. Some of the point source problems can be traced to inadequate safety measures relating to production, utilization, and disposal of many inorganic and organic compounds. A number of contaminants also arise from frequently used materials and reactions: coal and oil combustion, gasoline combustion, detergents, disinfectants, drugs (including endocrine disruptors), fertilizers, herbicides, and pesticides. Many contaminants can arise from Mother Earth, e. g., manganese, radionuclide’s, and arsenic. An overwhelming majority of water-quality problems are caused by diffuse nonpoint sources of pollution that are more difficult to monitor effectively. Often results from contamination by animal and human waste that is due to poor sanitary practices. Because of these problems, an estimated 1.2 billion people in the world drink unclean water daily [7-8].
2. Principles of Green Chemistry
- Green chemistry, an approach to the synthesis, processing and use of chemicals that reduce risks to humans and the environment, covers the following areas: √ Application of innovative technology to established industrial processes.√ Development of environmentally improved routes to important products.√ Design of new green chemicals and materials.√ Use of sustainable resources and biotechnology alternatives.√ Methodologies and tools for evaluating environmental impact.
2.1. Green Chemistry - An Initiative towards Solution
- Green chemistry solutions need to be found to resolve water pollution problems. First and foremost, these solutions should emphasize effective safety measures that prevent point source pollution. In addition, our vigilance to avoid nonpoint source pollution has to be appreciably improved. Realistically, water pollution in modern society seems inevitable. An effective way to deal with pollution problems is to use the least harmful ways to eliminate contaminants. The main objective of green chemistry is to promote research, development, and implementation of innovative chemical technologies that accomplish pollution prevention in a scientifically sound and cost-effective manner. Green chemistry solutions should highlight effective safety measures that prevent point and nonpoint-source pollution. Listed below are some of the principles of green chemistry that can be applied to solving water pollution problems [8].a. Minimize waste: Ideally, no waste should remain that requires treatment or clean-up.b. Usage of catalysts: Use catalytic rather than stoichiometric reactions that use excess reagents, and work only once.c. Increase energy efficiency: Run chemical reactions at ambient temperatures and pressures, when possible.d. Usage of safe solvents: Avoid using unsafe solvents, separation agents, or other auxiliary chemicals. Use innocuous chemicals if it is necessary.e. Analyze in real time: Include in-process, real-time monitoring and control during syntheses to minimize or eliminate the formation of by-products.f. Design chemicals and products that degrade after use: To prevent accumulation in the environment, design chemical products to break down to innocuous substances after use.There are different types of principles are implemented for the Green chemistry.1. Prevention: It is better to prevent than to clean or to treat afterwards (waste/pollution). This is a primary principle. The preventative action can change severely many attitudes among scientists developed in the last decades. Most of the chemical processes and synthetic routes produce waste and toxic secondary substances. Green chemistry can prevent waste and toxic by-products by designing the feedstock’s and the chemical processes in advance and with innovative changes.2. Maximum synthetic methods i.e. Atom Economy: All synthetic methods until now were wasteful and their yields between 70-90%. Green Chemistry supports that synthetic methods can be designed in advance to maximize the incorporation of all reagents used in the chemical process into the final product, eliminating the need recycling the by-products.3. Less hazardous chemical synthesis: Green Chemistry must strive wherever practical, to design safer synthetic methods by using less toxic substances as well as the products of the synthesis. Less toxic materials mean lower hazardous to workers in industry and research laboratories and less pollution to the environment.4. Designing safer Chemicals: Designing must become a fundamental aim of Green chemists to effect the desired function and properties of the chemical product while minimizing their toxicity to human and the environment. At present, there are around 100,000 chemical substances and materials in the market. 5. Safer solvents and auxiliary substances: Solvents, separation agents and auxiliary chemicals used in synthetic chemistry must be replaced or reduced with less toxic chemicals. Green chemistry initiated big changes in chemical laboratories and in the last decade there are less toxic solvents in chemical laboratories and alternative techniques.6. Design for energy efficiency: Chemists must recognize that until now there was very little thought to energy requirements in chemical synthetic chemical processes. Designing more efficient methods is a necessity and if possible synthetic methods should be conducted at room temperature and pressure to reduce energy requirements.7. Use of renewable raw materials and feed stocks: Starting raw material for synthetic processes are mostly petrochemical substances and products of refining. Raw materials must have very low toxicity and if possible to be renewable, rather than depleting. We know that there are many practical problems in finding renewable raw material.8. Reduce intermediate derivatives: Chemists must aim for reducing unnecessary derivatizations use additional reagents, are wasteful and produce large amounts of by-products and waste. The principle reminds chemists to change their old ways of producing chemicals with more chemical steps and additional materials. Designing new chemical synthetic routes are desired. 9. Catalysis, catalytic reagents: The use of catalysts is well known that can change dramatically the efficiency of chemical reactions and the yields of products. Catalytic reagents with great selectivity can be superior to stoichiometric reagents. New catalysts and more emphasis on catalytic processes is the future of green chemistry techniques. 10. Design products which degrade easily: Most chemical products and consumer items do not degrade very easily, thus causing environment problems. Green chemistry aims at designing products so that at the end of their useful life to break down into innocuous materials. Persistence into the environment is a negative aspect of many consumer products and this can be reversed by designing products which degrade in a short time.11. Inherently safer chemistry for accident prevention: Raw materials and chemical substances used in chemical process should be inherently safe, i.e. their properties and their degradation products to be non-toxic and not dangerous (e.g. to explode, to be flammable allergic to humans, cause burns to skin, etc.). Green Chemistry aims to stop the use of dangerous materials for the health and safety of workers and the consumer.These principles are clearly very difficult to apply immediately for many chemical processes. After twenty years of Green Chemistry initiatives and industrial applications it is amazing to see many creative innovations at various scientific and industrial processes. The cooperation of chemists, engineers, scientist, bio-scientists and technologist has achieved attractive results [9-10].Separation technologies and various chromatography proposals a variety of interesting methods to elimination of pollutants that meet up green chemistry requirements. In addition, chemical reactions that meet these requirements can be conducted, when necessary, to minimize the concentration levels of various toxicity of a given pollutant. Whenever by conduct chemical reactions to remediate pollution, it is important to assure that the overall impact is minimization of toxicity of the resultant products. An effective way to deal with water pollution problems is to use the least harmful ways to eliminate contaminants [11].
3. Developing Countries and Green Chemistry
- In developing countries, the beginning of green chemistry is still in a stage of immaturity, despite the significant need and the significant role green chemistry can play. Many of the practices in developing countries are still far from the concepts of safety, pollution prevention and design of energy efficiency. Environmental pollution and waste generation are some of the aching problems many developing countries are suffering from. Many of the reasons behind these problems lie in policies and strategies adopted that are based on end-of pipe treatment, rather than pollution prevention at source or implementing life cycle thinking in handling waste problems. Most often, income generation activities are dependent on an efficient use of energy and other resources such as water, which may pose some serious problems to future generations.The United Nations exposure on the millennium development goals at a country level indicated a high level of energy expenditure and limited energy resources in most of the developing countries. The report recommends the imperative need to portion the use of energy resources in these countries and to implement energy conservation policies. The similar tendency of difficulties developing countries face has been illustrated in the series of country reports produced by the rural development at the water and environment division of the World Bank. Sustainable chemistry could play a pivotal role in salvaging many of the ailing conditions that many of the developing countries are subjected to. The use of solar energy, introduction of sustainable farming, recycling, and the implementation of life cycle thinking and life cycle analysis as a management tool for some of the chronic issues such as municipal waste management, are some few examples of how green chemistry can benefit developing communities.Green chemistry can also have a very strong impact on water sufficiency issues in that part of the developing world where water resources is the most vital issue. It is through the implementation of cleaner production and use of safe and biodegradable chemicals that a huge volume of wastewater could be reused to quench the emerging, significant need of water in many of these countries.
3.1. Indian Scenario
- The green chemistry wave has reached our country too. Collaborations between industrial and academic partners are important to expedite the transfer of significant green products to the marketplace. For such collaborations to be successful, individuals in these two differently motivated cultures need to work together to advance green science. Governments could undoubtedly facilitate formation of more effective industrial/academic partnerships. Under an agreement with the Green Chemical Institute, University of Delhi has been accepted as an international chapter. The Indian chapter will promote green chemistry through education, information collection and dissemination, research and international collaboration via conferences, workshops, meetings and symposia [12]. In India, although there is growing awareness about the ill effects of pollution, promotion of continual introduction of environmentally friendly products a methodologies in the chemical industry needs to be furthered. Usage of nonconventional technologies is highly popular in India. First in this list is the usage of microwaves. Further, the microwave chemists are turning their attention toward microwave-assisted dry-media reactions in order to minimize solvent usage, an added advantage to already established microwave chemistry. In addition to microwave-assisted reactions, ultrasonic and photochemical reactions are also used as nonconventional reaction technology. Analytical chemistry has been at the centre of the green chemistry movement. Advances in analytical chemistry are major role into an environmental protection. In India, the focus for analytical chemistry is mainly on extraction technologies such as solid phase, ultrasound and microwave, supercritical fluid extraction, and automated soxhlet extraction. Monitoring and analysis of heavy metals and pesticides is very important for an agro economy-based country like India and chief governmental institutes like the Indian Agricultural Research Institute (IARI) and the Defence Research and Development Organization (DRDO) are working extensively in this field. Further removing of these elements from industrial and agrochemical usage is of prime importance for these [13].
3.2. Non-Academic Endeavour - A Major Concern
- Industry in India still needs to make major improvement from the environmental point of view. Most of the industrial R&D is mainly concerned with cost efficiency rather than eco-effective methods. Although there has been some joint work between academia and industries, still there is plenty opportunity for increased collaboration. There is instantaneous need for technology transfer from academic labs to industrial plants for meaningful application of green research. The best examples are the applications of enzymes in various industries ranging from drugs to leather. The textile industry is one of the highly revenue generating industries in India, and they are now switching over to microbial decolorization and degradation. There is an increasing need of exploring biodiversity for natural dyes and developing eco-friendly methodology for synthetic dyes. All these require more funding in the R&D of respective fields and greater interaction and coordination between industry, academia, and government.
4. Outstanding Government Initiatives
- Government can do a lot of good for the cause of green chemistry by increasing public alertness and by bringing and enforce strict environmental legislations. One of the fresh and notorious examples of government initiative is the conversion of diesel vehicles to compressed natural gas (CNG) in order to reduce pollution. Relocation of industries into industrial areas away from residential parks is another bold step taken by the government. Further, the government is also concentrating on new projects such as fuel pellets from municipal waste, aspirated H-cylinder engines for light commercial vehicles (LCVs), meeting India 2000 emission norms, battery-powered cars for pollution-free driving, hydrogen energy and energy towers for new environment-friendly fuel, development of traditional herbal drugs as adaptogens immune-modulators. The government should also increase funding to encourage research in green chemistry [14]. By introducing green chemistry education at all levels, the government can build a solid foundation toward green chemistry in India.
4.1. Remediation of Arsenic-Contaminated Water
- A large number of approaches have been investigated for removing arsenic from drinking water. Several useful identifications relating to the problem and its remediation have been published. Various existing and emerging arsenic-removal technologies are listed here.√ Coagulation with ferric chloride, alum, or natural products√ Sorption onto activated alumina√ Sorption onto iron oxide–coated sand particles√ Granulated iron oxide particles√ Polymeric ligand exchange√ Nanomagnetite particles√ Sand with zero-valent iron√ Hybrid cation-exchange resins√ Hybrid anion-exchange resins√ Polymeric anion exchange√ Reverse osmosis
4.2. Pollution Control Strategies
- Pollution can be defined in several ways. It has been recognized for some time now that most of the pollution is with respect to air, water and soil which are the most essential ingredients for life in this earth. It has also been recognized that the present day pollution that we generally conceive is mainly generated by the anxiety of human beings for their comfort. It is no doubt that the pollution has to be completely avoided if that were not to be possible, at least it should be reduced considerably. Each pollutant that has been introduced in the surroundings has its own health risk profile, which makes summarizing all relevant information into a single unit difficult. That is why we have decided to make a series of presentations on various manifestation of this matter. However, public health practitioners and decision makers in developing countries need to be aware of the potential health risks caused by air and water pollution and to know where to find the more detailed information required handling a specific situation. With this objective in mind we have attempted in this compilation some of the aspects of pollution and its control. The presentations are based on some awareness that sector wise the problem of pollution can and may be controlled.However this is not the only methodology one has to adopt. Climatic changes occurring on earth has been addressed in various forms like Governmental legislation, also from the point of view of disaster management and also from the points of view individuals/organizations to safeguard the interests of future generations. The climatic change has been mainly though not exclusively, considered to be due to carbon dioxide emission and methodologies based on carbon dioxide control or conversion have been evolved. Details of converting carbon dioxide to useful and value added products have been well addressed in literature. The approach for automobile exhaust control has been evolved in the form of the catalytic converter. The challenges in this converter are concerned with dealing with opposing oxidation (of carbon monoxide and hydrocarbons) and reduction reaction (reduction of NOx to N2) simultaneously and under varying experimental conditions of high space velocity, temperature and lean and rich Air/fuel ratio. Though three way catalyst as a concept has been introduced to carry out all these three reactions there are still challenges to be faced in terms of high cost of the noble metal catalysts employed, the operational difficulties that are introduced by the inclusion of a catalytic converter. Scientists, in their concern to publicize their innovations and discoveries, have been pushing hard their achievements too strongly, without much consideration to the after effects that will follow in the years to come. This has been true in each of the scientific discoveries that have broken in the last few decades. The environmental pollution is mainly the result of many processes and chemical reactions that are being practiced in chemical industries for manufacture of commodities and also due to indiscriminate use of energy sources. It is therefore necessary to understand the unit operations of these chemical industries. The proper understanding of the various unit operations of these chemical industries may provide us some conceptual frame work to combat the pollution of the environment. Human beings in their concern to present themselves with simple easy to adopt solutions to the issues with respect to contented living have come up with some possibly undesirable solutions that are not naturally degradable and hence leave some non-degradable marks in nature and this aspect has been addressed at various stages but no viable solution has been found yet meeting the human desire for low cost, simple and elegant methodology [15].
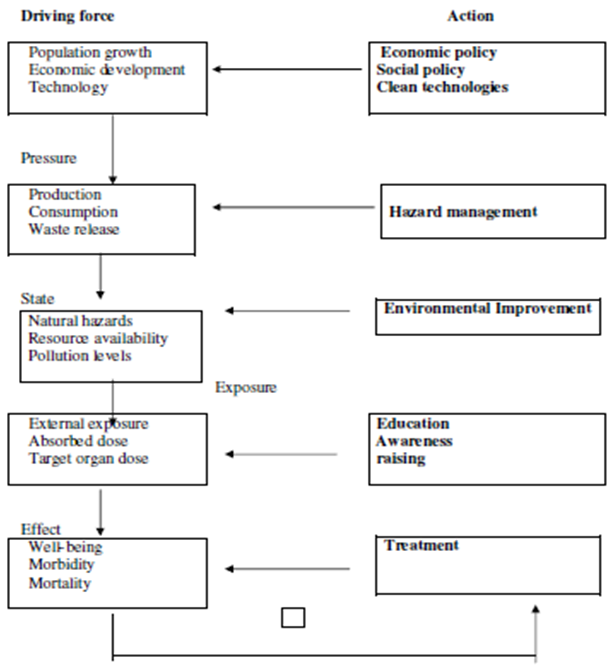
5. Green Chemistry has Advanced from Theory to Practice
- As we can see from all the above technological advances, Green Chemistry principles have advanced considerably in the last decades. Research on various industrial applications have been very successful and with considerable advantages for energy consumption, less toxic products and minimum waste. These advances have contributed first of all in the safely and health of workers who work in chemical industries, making of products with basic materials workshops and other professional people involved in the transport and distribution of these products. Secondly, green chemistry found alternative ways to cut energy consumption, or by changing processes, or through new catalytic routes, in order to save energy [16-18].
6. Conclusions
- Water pollution in our present society is inevitable. Effective safety measures that prevent point-source pollutions should be employed. In addition, our vigilance to avoid nonpoint-source pollution must be significantly improved. Green chemistry solutions should be employed, wherever possible. An effective way to agreement with the water pollution problem is to use the least harmful ways to eliminate contaminants. Chromatography and separation chemistry offer a variety of interesting approaches to achieve these goals. Interesting examples of remediations have been provided to help correct arsenic contamination of groundwater, which affects nearly 200 million people worldwide. A variety of novel methods for prevention and remediation of water pollution that entail radical reaction and utilization of nano-particles have been considered.The role of the academia is to bring about a mass understanding about the pertinence of Green chemistry. This body must also take upon itself to device appropriate educational material for different levels of curricular instructions. The research and development and the science and technology agencies that are responsible for the funding of scientific activities in the country must encourage and give preference to the development of greener science and technology. In order to ensure global environmental protection while keeping scientific and economic development on the forefront, the policy makers should understand the role of ‘green’ science and technology and make pollution prevention, rather than pollution control, their slogan. Though it is true that many industries and research organizations are yet to implement the principles of Green chemistry, nevertheless some of them have begun to realize that the ‘think green’ culture is more than just a fashion. In fact the most successful chemistry researchers and chemical technologists will like to appreciate and apply the values of Green chemistry in innovation, application and teaching.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML