-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(3C): 12-18
doi:10.5923/c.materials.201502.03
Photocatalytic Behaviour of Nanocomposites of Sputtered Titanium Oxide Film on Graphene Oxide Nanosheets
Sunil Meti , Udaya Bhat K. , Rahman M. R. , Jayalakshmi M.
Department of Metallurgical and Materials Engineering, NITK Surathkal, Srinivasnagar, India
Correspondence to: Udaya Bhat K. , Department of Metallurgical and Materials Engineering, NITK Surathkal, Srinivasnagar, India.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In this investigation, fabrication, characterization and photocatalytic properties of graphene oxide nanosheets (GONs) and titanium oxide (TiO2) thin film composites, has been reported. Graphene oxide nanosheets were prepared by using a bottom-up approach, namely, hydrothermal route. Sucrose and de-ionized water were the constituents and synthesis was carried out by using an autoclave. GONs were scooped on a glass substrate. GON-glass substrate combination was annealed in argon to restore the conductivities. Deposited GONs were coated with TiO2 using reactive magnetron sputtering. The as-deposited TiO2 was amorphous and another annealing treatment was given to convert it into crystalline phase. The composites were characterized using X-ray diffractometry (XRD), Fourier transform infrared spectrometry (FTIR), Scanning electron microscopy (SEM), Atomic force microscopy (AFM), Raman spectroscopy and Transmission electron microscopy (TEM). Their photocatalytic properties were investigated by using UV-Visible spectroscopy. Photocatalytic behaviour was compared with bare TiO2. Methyl orange solution was used as a model solution. It was observed that GONs provided a good support for TiO2. The graphene oxide layer enhances the photocatalytic activity of TiO2 by at-least three times compared to bare TiO2.
Keywords: Graphene oxide, Hydrothermal route, Bottom-up synthesis, Sputter deposited TiO2 films, Annealing
Cite this paper: Sunil Meti , Udaya Bhat K. , Rahman M. R. , Jayalakshmi M. , Photocatalytic Behaviour of Nanocomposites of Sputtered Titanium Oxide Film on Graphene Oxide Nanosheets, American Journal of Materials Science, Vol. 5 No. 3C, 2015, pp. 12-18. doi: 10.5923/c.materials.201502.03.
Article Outline
1. Introduction
- Composites containing carbon nanotubes (CNT’s), graphene and its oxide (GO) show unique properties such as, theoretically high charge carrier mobility and surface area [1]. Graphene oxide (GO) is environmentally friendly and cheap and hence, vastly studied in recent years to apply it in almost each and every field [2]. The carbon nanotubes have the capability of string one electron per 32 carbon atoms in their conjugated sp2 networks [1]. Titanium oxide (TiO2) has excellent chemical stability, mechanical hardness and optical transmittance with high refractive index. Hence, titanium oxide thin films are promising in the fields, such as, solar cells, optical and protective coatings. TiO2 thin films are being considered as the most promising materials in environmental cleaning such as photocatalytic purifier and photochemical solar cells [3]. In this context, nanocomposites of GO and TiO2 are highly promising. These nanomaterials owing to their physical and electronic properties have made significant contributions in the research of photocatalytic enhancement. Focus is also on enhancing light absorption range of TiO2 (which is otherwise limited to only UV region) and overcoming the problem of fast recombination of photogenerated charge carriers [1]. There are different routes to synthesize graphene and convert into graphene oxide (GO). Few of them are chemical vapour deposition (CVD) [4], SiC pyrolysis [5], graphite oxide reduction [6], mechanical exfoliation method (also called the‘‘Scotch tape method’’) [7] etc. In this paper, design, fabrication, and performance of model nanocomposites of graphene oxide–TiO2 thin film with an aim to understand the role of graphene oxide prepared using the Tang Lau method via hydrothermal route has been reported. This method was selected due to ease of synthesis and requirement of mild chemicals compared to top-down approach. Also, this can produce relatively coarse graphene oxide nanosheets (GONs) [8]. TiO2 thin films can be prepared by different techniques such as sol-gel process [9], chemical vapour deposition (CVD) [10, 11] sputter depositions [1], and ion beam-assisted processes [12]. In this investigation DC magnetron sputtering technique was used for the deposition of TiO2 thin films.
2. Experimental Section
- Glass (soda lime glass plates, dimensions: 10×2.5×0.5 cm) and aluminium foils were used as substrates for deposition. The substrates were cleaned by repeated ultrasonication, washing in acetone and by drying. Graphene oxide nanosheets (GONs) are grown by using hydrothermal route [8]. A solution of 0.5 molarity was prepared by using sucrose (make: Sigma-Aldrich) and deionised water. 50 ml of prepared solution was poured into a Teflon lined autoclave and kept in an oven at a temperature 180 ± 1°C for time duration of 90 minutes. After the set time, the autoclave was cooled to room temperature, GONs were scooped and then transferred onto substrates (either glass or aluminium foil) and annealed. The synthesis parameters were selected from the work of Tang et al. [8].
2.1. Annealing
- Annealing of GONs was carried out under argon atmosphere at 1 cubic centimetre per minute (ccpm), at 450 °C for 5 minutes [8]. To minimize oxidation of the GONs during heating, GONs and substrate were wrapped with Cu foils. Upon increase in temperature, the edge-plane–COOH (carboxyl) groups become highly unstable, whereas >C=O (carbonyl) are more difficult to be removed. The most stable among the oxygen species is C-OH (hydroxyl) group in the GONs. Annealing of GONs at a temperature greater than 400°C leads to decomposition of –COOH group. Changes during annealing treatment are schematically shown in Fig. 1. For nanoelectronics applications, the annealing temperature should cross 1000°C [13].
 | Figure 1. Presence of traces of phenolic groups after annealing GON in presence of argon at 450°C [13]. |
2.2. Sputtering
- Glass substrate with coated graphene oxide was used as substrate for TiO2 thin film deposition. TiO2 thin film was deposited by DC reactive magnetron sputtering method (360 V, 0.35 A). The deposition was performed at room temperature in vacuum of 3×10-5 mbar and for 45 minutes. The oxygen and argon flow rates were set as 4 ccpm and 30 ccpm, respectively. Annealing of TiO2 was carried out at 450°C in argon atmosphere for 2 hours to convert amorphous TiO2 into the predominantly anatase structure [1, 14]. Both deposition time and annealing promote formation of anatase phase. The annealing parameters for sputtered TiO2 were narrowed down as in [1].
2.3. Photocatalytic Experiment Using Methyl Orange
- Photocatalytic experiments were carried out at 25°C in a chamber which is positioned with a UV source lamp with maximum power of 32 W. A glass cell containing 5 µM solution of methyl orange (MO) in de-ionized (DI) water, is used as test sample [15]. The solution is magnetically stirred during photocatalytic experiment. Glass substrates with GON-TiO2 composite and bare TiO2 films (after heat treatment) were dipped in the bath solution separately and exposed to UV light for 5 hours duration. The effective area of the TiO2 film / composite, used for photocatalytic experiment was 10 cm2. The concentration of the methyl orange before and after exposing it to UV was estimated using UV-Visible spectrometer and they are used for estimating photocatalytic degradation [16, 17]. If Co and Cf are initial and final concentration of methyl orange in the solution, photocatalytic degradation is given by equation (1)
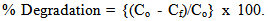 | (1) |
3. Characterization Techniques
- Phase identification in the samples was carried out by using X-ray diffraction (XRD, machine-JEOL-JPX 8) technique using copper target (1.54Å). A scan rate of 0.5° min-1 was used. The structure of the synthesized GONs was studied using Fourier transform infrared (FTIR, machine-JASCO-4200) spectrometer using KBr pellets. Band energy was studied using Raman spectroscopy with 325 nm and 514 nm LASER (machine-Horibajobin Yvon-LabRAM HR). The thickness and roughness (topography) of the samples were characterized by an Atomic force microscope (AFM, machine-A.P.E. Research-A100). The absorption spectrum of GONs and bare TiO2 film were obtained separately using UV-Visible spectrometer (machine-Analytikjena-S600). The average transmittance of the sputtered film in the visible region is ~80%. Scanning electron microscope attached with an energy dispersive spectroscope (SEM, machine-JEOL-JSM-6380LA, equipped with EDS from Oxford instruments) was used to investigate chemical composition of the composite. Transmission electron microscope (TEM, machine-JEOL-JEM-2100) images were taken to study the morphology of GONs.
4. Results and Discussion
- Fig. 2 shows XRD plots of bare GONs and TiO2 film (after heat treatment) separately. The XRD plot of GON [Fig. 2 (a)] shows reflection corresponding to (001) plane at 10.06°. Predominant (001) reflection also indicates that the synthesized GON has layered structure. The calculated interplanar spacing is 0.87 nm against a reported value of 0.80 nm [12]. Similarly, the XRD plot of a TiO2 film [Fig. 2 (b)] indicates that TiO2 has anatase phase (data file No JCPDS FILE: 21-1272). Formation of anatase phase after heat treatment of sputtered TiO2 is reported by Ghasemi et al. [18]. During heat treatment, anatase predominantly grows along (101) orientation.
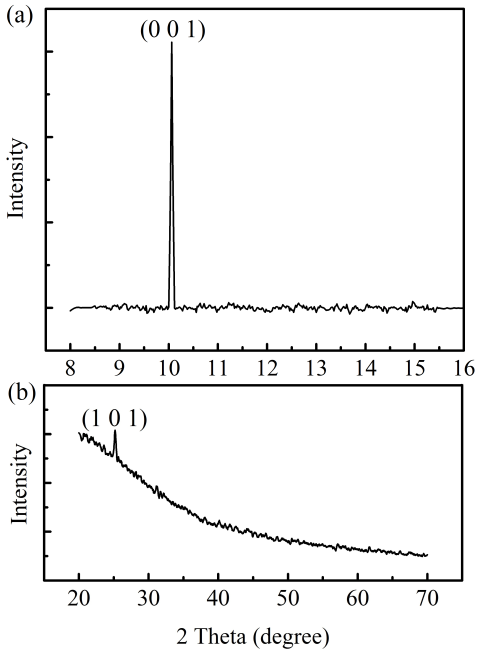 | Figure 2. XRD graph of (a) GO and (b) TiO2 sputtered film |
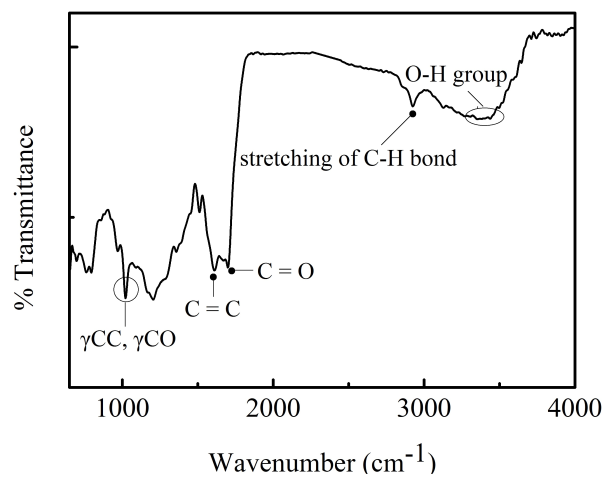 | Figure 3. FTIR spectrum of graphene oxide |
 | Figure 4. Raman spectrum of graphene oxide and TiO2 film |
 | Figure 5. (a) AFM images of graphene oxide, (b) Roughness scans along two lines marked 1 and 2 in Fig. 5(a) |
 | Figure 6. (a) UV- Vis spectrum of graphene oxide, (b) UV- Vis spectrum of TiO2 film |
 | Figure 7. SEM micrographs of (a) GON sheet, (b) GON annealed sheet (c) GO/TiO2 film and (d) GO/TiO2 annealed film |
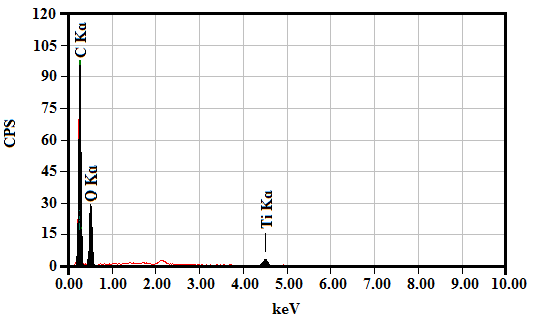 | Figure 8. EDX spectrum of GON and TiO2 film |
 | Figure 9. TEM micrographs of graphene oxide nanosheets |
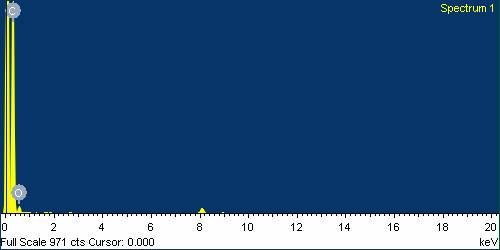 | Figure 10. TEM EDX pattern of GONs |
 | Figure 11. UV-Vis spectra indicating extent of degradation of methyl orange solution |
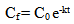 | (2) |
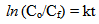 | (3) |
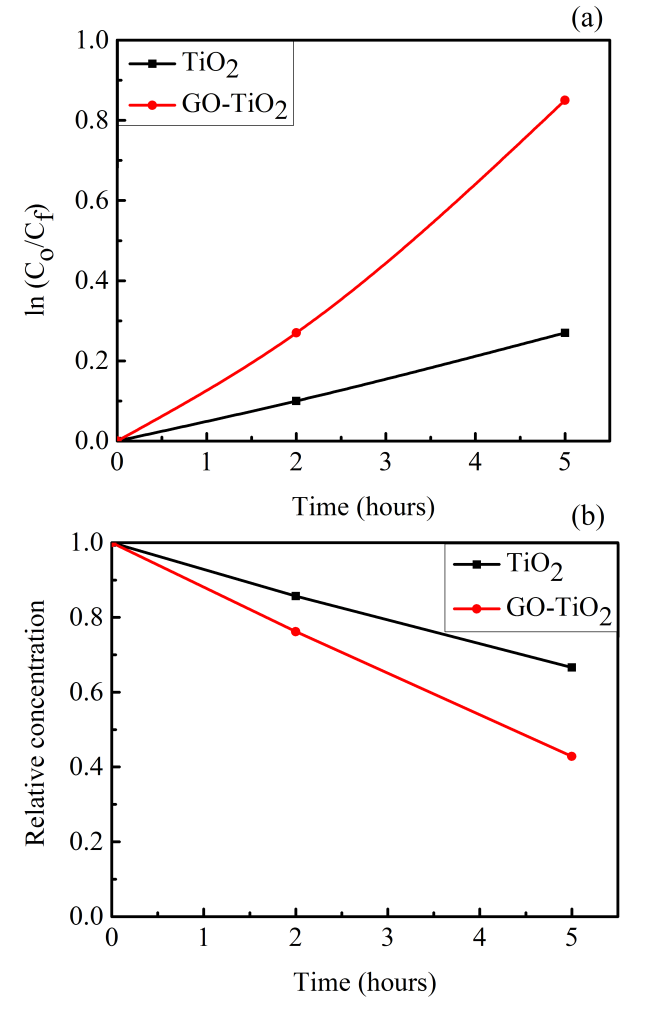 | Figure 12. (a) Plot indicating a pseudo first order reaction (b) relative concentration of methyl orange at different time scales |
5. Conclusions
- Graphene oxide nanosheets-TiO2 thin film nanocomposites were prepared using a combination of hydrothermal route and DC magnetron sputtering. Volatile species were minimised by a set of annealing treatment. The graphene oxide nanosheets were relatively coarse and surface roughness was negligible. The samples were characterised for their bonding behaviour by using Raman spectroscopy, FTIR and UV-Vis spectroscopy. Their ability to degrade methyl orange was tested and observed that the layered composite shows three times improved behaviour compared to bare TiO2 thin film, deposited and annealed under similar conditions.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the help rendered by Dr. Anandhan S., Met. & Matls. Engg. Dept., NITK Surathkal in FTIR spectroscopic studies, Dr. Sathyanarayana, Physics Dept., NITK Surathkal in annealing treatments and Dr. Ramachandra Bhat, Chemistry Dept., NITK Surathkal in UV-Vis spectroscopic studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML