-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(3B): 21-24
doi:10.5923/c.materials.201501.05
Chronic Sodium Arsenite Exposure Effects Cognitive Behaviour of Sprague Dawley Rats
Saima Ashraf, Zalina Ismail
BRAINetwork Center for Neurocognitive Science, School of Health Sciences, Universiti Sains Malaysia, Kubang Kerian Kelantan, Malaysia
Correspondence to: Saima Ashraf, BRAINetwork Center for Neurocognitive Science, School of Health Sciences, Universiti Sains Malaysia, Kubang Kerian Kelantan, Malaysia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The present study was performed to investigate the chronic effects of sodium arsenite on cognitive behaviour of male Sprague Dawley rats. Mature male rats at the age of 90 days were divided into 4 groups of 8 animals each. Group I received saline water whereas the other three groups received sodium arsenite at doses of 3, 5 and 8mg/kg of body weight respectively, for 60 days by intraperitoneal injection. The Morris Water Maze test was performed for spatial memory at day 60. Following this, the animals were then dissected and their hippocampi were collected for histological studies. It was found that the effect of chronic exposure was dose dependent. A significant decrease (p≤0.05) was observed in cognitive behaviour in the higher dose groups 5mg/kg and 8mg/kg) in comparison to the control group. Histological evaluation revealed dose-dependent, gradual damage in histoarchitecture of hippocampus. Moreover no significant change was observed in any experimental parameter in the low dose group (3mg/kg) in comparison to the control group. The results revealed that sub chronic exposure to sodium arsenite caused degenerative changes in hippocampus and effects spatial memory in a dose dependent manner. Chronic exposure to arsenic causes neurotoxicity in male Sprague Dawley rats.
Keywords: Sodium arsenite, Morris water maze, Cognitive behaviour, Spatial memory, Sprague Dawley
Cite this paper: Saima Ashraf, Zalina Ismail, Chronic Sodium Arsenite Exposure Effects Cognitive Behaviour of Sprague Dawley Rats, American Journal of Materials Science, Vol. 5 No. 3B, 2015, pp. 21-24. doi: 10.5923/c.materials.201501.05.
Article Outline
1. Introduction
- Arsenic is a naturally occurring element that ubiquitously exists in both organic and inorganic form in the environment Arsenic is rarely found in a pure state; rather, it exists in both trivalent and pentavalent oxidation states as a chemically unstable sulfide or oxide, or as a salt of sodium, potassium, calcium. Human exposure to arsenic is a significant public health concern. The ubiquitous distribution of arsenic (a metalloid) in the soil, air and water [1] makes it an environmental contaminant of global concern. Arsenic affects all organ systems of the body including central nervous system (CNS). In the nervous system seizures, encephalopathy, peripheral neuropathies, and behavioral changes have been reported. [2] Arsenic has neurotoxic effects on both central and peripheral components of the mature nervous system. There is increasing evidence that exposure to arsenic is also toxic to the developing nervous system and can result in decreased cell division and increased apoptosis in cultured developing neurons. Sodium Arsenite exposure induces neurotoxicity by initiating destabilization and interruption of the cytoskeletal framework, ultimately leading to axonal degeneration. [3] Arsenic-induced oxidative stress in the brain causes oxidative DNA damage and subsequent brain cell death and induces the degeneration of dopaminergic neurons resulting in Parkinson like symptoms. [4] Acute arsenic toxicity decreases acetyl cholinesterase activity and hence causes cholinergic crisis like situation with altered mental status and weakness, which can be associated with peripheral neuropathy, neuropsychiatric abnormalities, and extrapyramidal disorders. [5] Moreover, arsenic affects the peripheral nervous system by disrupting the neuroskeletal integrity and thus markedly diminishes the nerve conduction velocity in the peripheral nerves to cause peripheral neuropathy. [6] The exposure to arsenic suppresses the NMDA receptors in hippocampus, which play a pivotal role in synaptic plasticity, learning, and memory, leading to neurobehavioral disorders and cognitive dysfunction. [7] The chronic arsenic exposure is associated with morphological changes in axons and nerve fibers of the striatum which disturbs central structural organization. [8] Hence, oxidative stress, induction of thiamine deficiency, and inhibitions of pyruvate decarboxylase, acetyl cholinesterase, reduction in biogenic monoamines seem to play a pivotal role in arsenic-induced neurotoxicity.The animal models of arsenic toxicity are associated with inconsistent neurotoxicity because of varying doses, duration, and different salts of arsenic used in various studies. However, these have been able to provide deep insight into pathophysiological mechanisms involved in arsenic induced neurotoxicity. Across-sectional study in San Luis Potosi, Mexico [9] examined the impact of arsenic, lead and under-nutrition on the neuropsychological performance of school children aged 6 to 9 years. Subjects included 41 children living within 1.5 Km of a smelter complex (Morales Zone) with increasing arsenic and lead concentrations, and 39 children living 7 Km upwind from smelter (Martinez Zone). The geometric mean total arsenic concentration in urine was higher in the Morales children than in the Martinez children. Maternal and paternal educational attainment, socioeconomic status was lower in the Martinez group. Neuropsychological performance was assessed using the Weschler Intelligence Scale for children, Revised version, for Mexico (WISC-RM). The Morales children scored significantly lower than the Martinez children on the full-scale IQ test and other neuropsychological sub scores.The aim of the present study was to assess the toxic effect of chronic exposure to sodium arsenite at different dose levels of sodium arsenite (3,5 and 8mg/kg) by studying the histopathological changes of hippocampus that effects spatial memory using male Sprague Dawley rats as an experimental model.
2. Materials and Methods
2.1. Experimental Animals
- Healthy adult male Sprague Dawley rats of 12 weeks of age and weighing between 350 and 400 g were procured from Animal House and Service Centre of Health Campus, University Sains Malaysia, Kelantan, Malaysia. They were maintained at the Animal House under controlled conditions (27 ± 2°C temperature; 40± 5% relative humidity). The animals were given standard rat pelleted feed and water ad libitum. The experimental protocols were approved by the Institutional Animal Ethics Committee.
2.2. Chemicals and Experimental Designs
- Sodium arsenite (NaAsO2) was purchased from Sigma Chemical Co. The different doses were selected on the basis of their concentration reported (WHO, 1999) in the water bodies of different regions of Bangladesh. The different doses were prepared in distilled water and then given by IP injection daily. The study was carried out for 60 days. The rats were divided into four groups of eight animals each. Group I received saline water and served as the control. Group II-IV animals received sodium arsenite at the dose level of 3mg/kg, 5mg/kg and 8mg/kg respectively for 60 days via IP injection.
2.3. Morris Water Maze
- The spatial learning was tested by using Morris Water Maze. Spatial learning and memory function in rats was assessed in the MWM by conducting two different tests hidden platform acquisition and probe trial test-according to Fan et al. [47] and Luo et al. [17] with some modifications.Training In the hidden platform acquisition test, the rats underwent 4 consecutive days of training in the MWM that was filled with tap water (2561uC) with two trials each day. In each trial, the rats were released from four randomly assigned release points (N, W, S, and E). The rats were placed in the tank, facing the wall of the pool, and allowed to freely swim to search for the escape platform for a maximum of 120s. The time to reach the platform was recorded as the escape latency. If a rat failed to find the platform within 120 s, then it was guided to the platform by a stick and placed on the platform for 30 s; in this case, the escape latency was recorded as 120 s for this trial.PROBE TESTAfter the hidden platform acquisition test, a probe test was conducted by removing the platform. The rats were allowed to freely swim in the MWM for 120 s and the following three indices were recorded (i) the time spent in the target quadrant where the hidden platform was previously located. (ii) The time of first crossing the location where the platform was originally located and (iii) the number of rats which passed through the original hidden platform location.The three indices, especially the time spent in the target quadrant, indicate the degree of memory maintenance and consolidation. After the 4-day training period, the rats were returned to their cages and allowed to rest for 1 week. These trained rats were then placed in the MWM again and tested under the same conditions to evaluate long-term memory ability.
2.4. Animal Sacrifice, Collection of Hippocampus
- On the last day of treatment, all the animals were sacrificed under light ether aesthesia. The hippocampus was dissected out quickly and fixed in bouins fixative for histological evaluation.
2.5. Statistical Analysis
- Values obtained as mean ± SEM were subjected to one-way analysis of variance (ANOVA) followed by Dunnet's test. The values of all the arsenic exposed groups were compared to the control group and the values at P<0.05 and P<0.01 were considered as significant.
3. Results
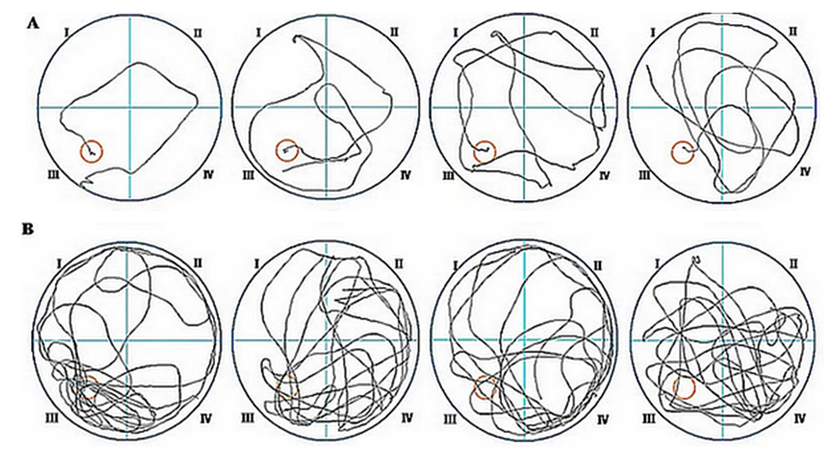
- The following figure 1 shows images of swimming tracks during training and probe test.
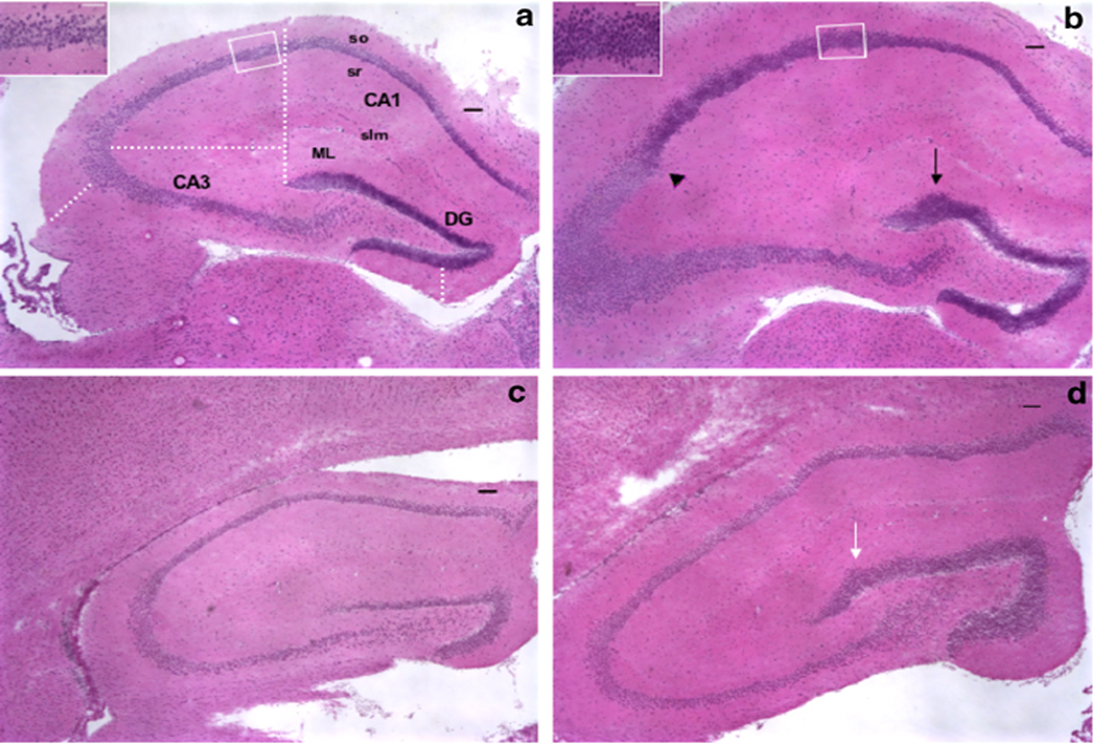 | Figure 2. Histology of Hippocampus CA1 and CA2 area. Slide a is control and b treated with 3mg/kg, c treated with 5mg/kg with and d with 8mg/kg of body weight are pathological slides |
4. Discussion
- The results showed that sodium arsenite level in blood and the hippocampus in rats exposed to dose of 5mg/kg and 8mg/kg for two months were significantly higher than in the low dose group 3mg/kg and control group. These findings indicate that excessive sodium Arsenite can cross the blood-brain barrier and accumulates in the brain in rats, which is consistent with previous studies [10].Sodium arsenite is a neurotoxicant that can impair cognitive capacity. Histopathological changes have been observed during this study that may be the cause of this impairment of spatial memory in rats. Our results were consistent with other epidemiological and experimental animal studies, in which sodium arsenite exposure was compared with control rats, where the rats that were exposed to high dose of sodium arsenite exhibited obvious delays in finding the hidden platform not only on the four training days but also on the 11th day. These results suggest that the effect is dose dependent and impair spatial learning and memory ability.These findings indicate that sodium arsenite exposure impair both short-term and long-term memory that is associated with decreased intelligence and learning and memory ability [11].
5. Conclusions
- In conclusion, sodium arsenite can cross the blood-brain barrier and accumulates in the brain in rats. The above research indicates that sodium arsenite exposure can impair child intelligence and impair neurobehavioral function, including learning and memory.This study was supported by Universti Sains Malaysai research cluster grant (1001/P/SKBP/8630021).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML