-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(3B): 16-20
doi:10.5923/c.materials.201501.04
Structural and Photoluminescence Studies of Rutile TiO2 Nanorods Prepared by CBD Method on Si Substrates
Abbas M. Selman1, 2, Z. Hassan1
1Nano-Optoelectronics Research and Technology Laboratory (N.O.R.), School of Physics, Universiti Sains Malaysia, Penang, Malaysia
2Department of Pharmacology and Toxicology, College of Pharmacy, University of Kufa, Najaf, Iraq
Correspondence to: Abbas M. Selman, Nano-Optoelectronics Research and Technology Laboratory (N.O.R.), School of Physics, Universiti Sains Malaysia, Penang, Malaysia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In this work, nanoflower arrays of rutile TiO2 composed of nanorods were fabricated on p-type (111)-oriented silicon substrates and, all substrates were seeded with a TiO2 seed layer synthesized by radio-frequency reactive magnetron sputtering system. Chemical bath deposition (CBD) was carried out to grow rutile TiO2 nanorods on Si substrate. Raman spectroscopy, X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM) analyses showed the tetragonal rutile structure of the synthesized TiO2 nanorods. Optical properties were examined by photoluminescence spectroscopy. The spectra exhibit one strong UV emission peak. Which can be seen at around 396 nm. In the visible region, TiO2 demonstrated two dominant PL emissions centered at around 528 and 705 nm. The experimental results showed that the CBD method enabled the formation of photosensitive, high-quality rutile TiO2 nanorods with few defects for humidity sensor and in future optoelectronic nanodevice applications.
Keywords: CBD, Nanorods, Rutile TiO2, PL Studies
Cite this paper: Abbas M. Selman, Z. Hassan, Structural and Photoluminescence Studies of Rutile TiO2 Nanorods Prepared by CBD Method on Si Substrates, American Journal of Materials Science, Vol. 5 No. 3B, 2015, pp. 16-20. doi: 10.5923/c.materials.201501.04.
1. Introduction
- One of the most important semiconductor materials is titanium dioxide (TiO2), a well-known semiconductor material with a wide band gap (3.02 eV for rutile and 3.20 eV for anatase [1, 2]. TiO2 exists in three crystal structures, including anatase, rutile and brookite. Rutile phase has unique properties such as high hardness, high Young’s modulus, high refractive index, high dielectric constant, excellent mechanical strength, high transparency in visible region, high ultraviolet (UV) absorption rate, and high chemical stability [3-6]. These properties increase the demand for the rutile form of TiO2 as a research material for various applications, including photo catalysis [7], dye sensitized solar cells [8], gas sensors [9], and UV detectors [10], because of their remarkable optical and electronic properties. TiO2 exists in three main crystalline structures phases, namely, anatase, brookite, and rutile, and each crystalline form exhibits different physicochemical properties [11]. Rutile has excellent properties both physically and chemically [12]. Fabricating nanostructures of TiO2, such as nanorods, is a representative example among the sustained efforts. Different deposition methods have been used to grow one-dimensional TiO2 nanostructures, such as chemical vapor deposition [13], hydrothermal [14], sol–gel method [15], thermal evaporation [16], and chemical bath deposition (CBD) [17]. Among these methods, CBD is a flexible technique and is a promising approach because this method does not require sophisticated instruments; the preparation parameters are easily controlled; the starting chemicals are commonly available and have low cost for TiO2 nanostructure synthesis by strongly controlling their morphology [12]. High quality rutile was successfully synthesized on Si (100) substrate with a ZnO buffer layer via radio frequency (RF) magnetron sputtering [13]. Rutile TiO2 nanorods are hydrothermally deposited on boron-doped diamond film with a ZnO buffer layer to increase the density and improve the morphology [14]. In the present work, the effects of duration time on growth of rutile TiO2 nanorods deposited onto p-Si (111) substrate were examined. All substrates were seeded with a TiO2 seed layer synthesized by radio-frequency (RF) reactive magnetron sputtering. CBD was carried out to grow rutile TiO2 nanorods on the Si substrate. The crystal structure, surface morphology and other characteristics of the deposited TiO2 nanorods were investigated by X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), photoluminescence (PL), and Raman spectroscopy.Experimental detailsThe rutile TiO2 nanorod synthesis has been described in our previous work [21]. In brief, CBD was used to synthesize the TiO2 NRs by heating an acidic solution of titanium (III) chloride containing the immersed seed layer (TiO2)-coated Si substrate. Titanium (III) chloride liquid was mixed with deionized water (DI water) in appropriate quantities. Exactly 4 ml of TiCl3 (15 wt.% in HCl; Merck Sdn Bhd, Malaysia) was added to 50 ml distilled water. Urea (NH2CONH2; 0.1 M) was added to adjust the pH to approximately 0.7. After stirring at room temperature (27°C) for 1 h, a homogeneous violet solution was obtained. The substrate was then vertically immersed in the aforementioned bath that was then heated at 55°C. The substrate coated with TiO2 NRs were removed after 2 h, rinsed with distilled water, and dried with nitrogen gas. To improve crystallinity, the film was heat treated at 550°C for 2 h in air. The structure and morphology of the prepared TiO2 NRs were characterized and analyzed by XRD (PANalytical X’Pert PRO MRD PW3040) with CuKα radiation (λ = 1.541 ˚A ) and FESEM (Leo Supra 50VP, Carl Zeiss, Germany) equipped with an energy-dispersive X-ray (EDX) system. Optical properties were measured at room temperature with a PL spectroscopy system (Jobin Yvon HR 800 UV, Edison, NJ, USA) having a He – Cd laser (325 nm, 20 mW). Electrical characterization was performed inside a sealed plastic chamber by a Keithley 2400 Sourcemeter. Tests were carried out under different RH at room temperature (25±1°C). The different RH conditions were set up by means of the mix between air bubbling in water and dry air. Inside the chamber, a commercial humidity sensor (LinPiccoTM A05 Basic, Capacitive Humidity Module) was used as a humidity indicator in order to control the different atmospheres.
2. Results and Discussion
- The XRD patterns of the prepared TiO2 NRs onto TiO2 seed layer-coated p-type (111)-oriented silicon substrate is depicted in Fig. 1. The scanning Bragg angle was within the 2θ range from 20° to 80°. Within this range, dominant sharp peaks were noted in the XRD pattern at 27.4° and 36.1° that confirms (110) and (101) planes growth respectively.
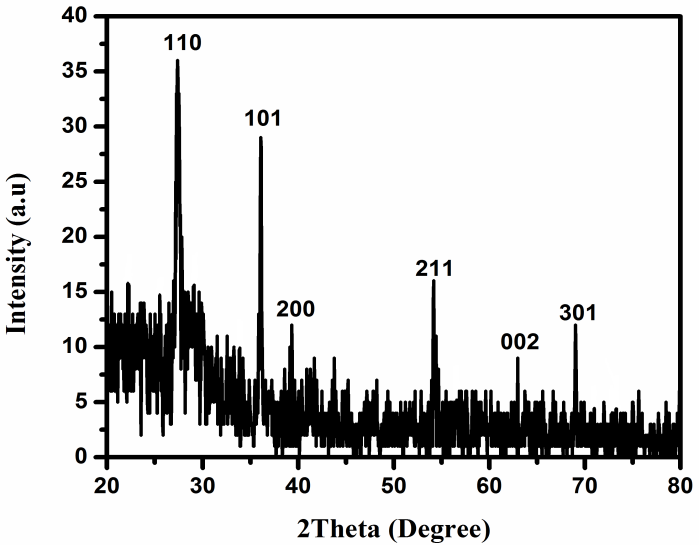 | Figure 1. XRD patterns of rutile TiO2 nanorods grown on silicon (111) substrate |
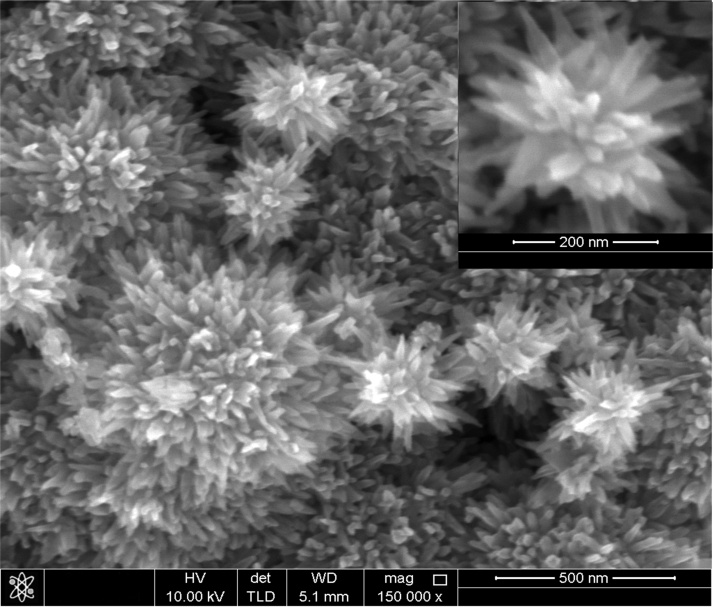 | Figure 2. FESEM image of the rutile TiO2 nanorods grown on silicon (111) substrate |
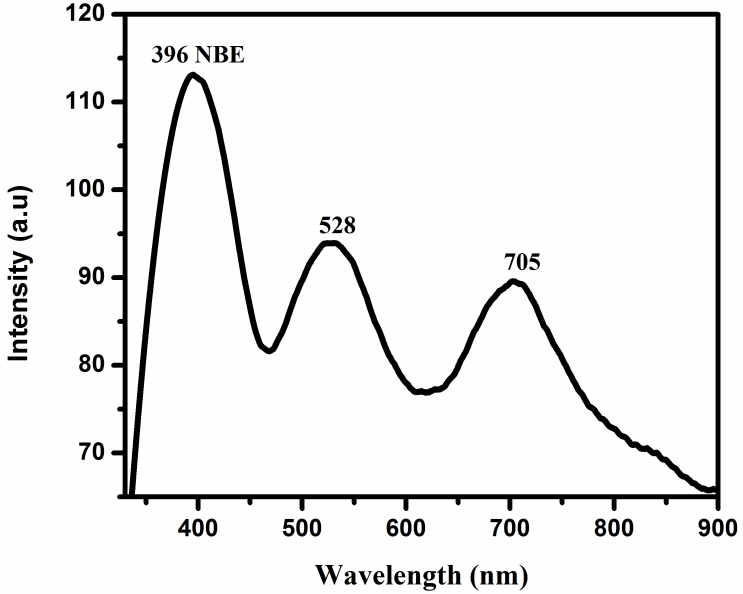 | Figure 3. PL spectra at room temperature of rutile TiO2 nanostructures grown on silicon (111) substrate |
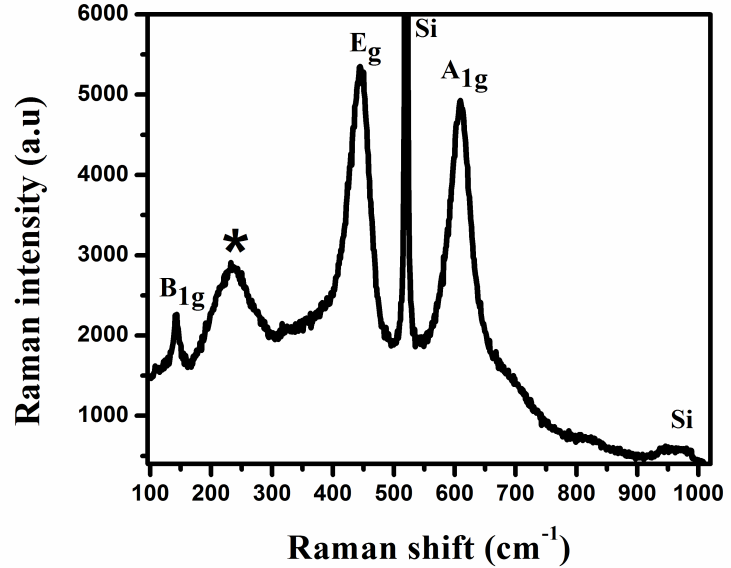 | Figure 4. Raman spectra of rutile TiO2 NRs grown on silicon (111) substrate |
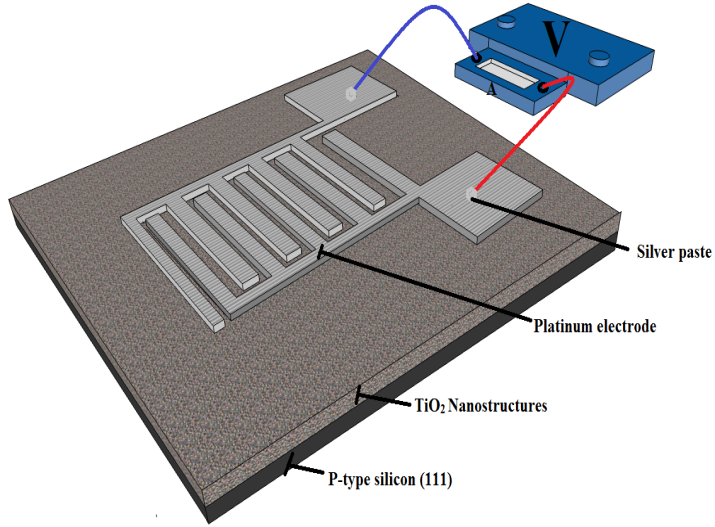 | Figure 5. The schematic diagram of the device (MSM-structured Humidity sensor) |
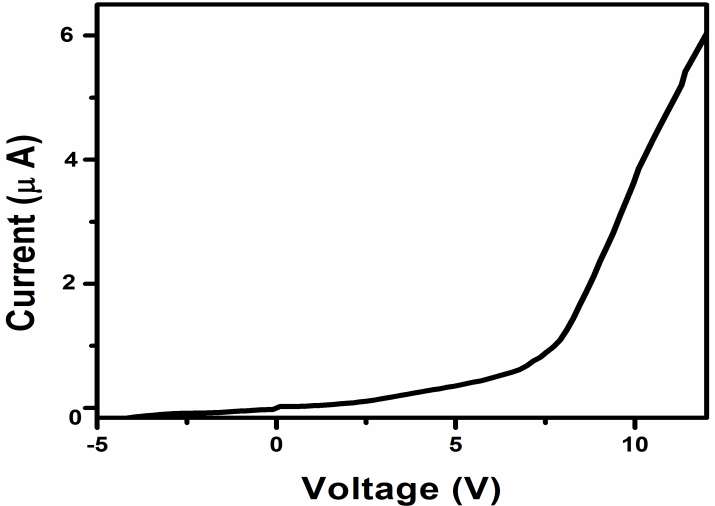 | Figure 6. Current-voltage characteristics of the (Pt/TiO2NRs/Pt) MSM-structured UV detector under dark |
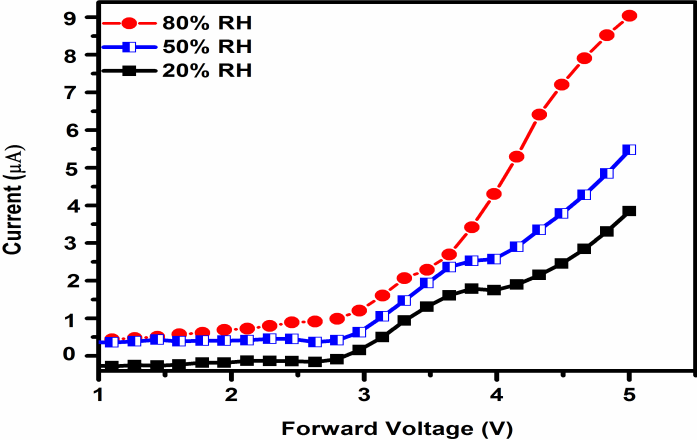 | Figure 7. Current–voltage characteristics of the devices at20%, 50% and 80% RH and RT |
3. Conclusions
- High-quality rutile TiO2 NRs were grown on p-type Si (111) substrate via chemical bath deposition method. Raman spectra depicted the rutile crystal phase of TiO2, and the highest PL UV intensity revealed the high optical quality of TiO2 nanorods with few defects. These films may have potential use for humidity sensor and in future optoelectronic nanodevice applications.
ACKNOWLEDGEMENTS
- This work was supported by FRGS grant (203/ PFIZIK/6711353), PRGS grant (1001/PFIZIK/846073) and Universiti Sains Malaysia.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML