-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(3B): 1-4
doi:10.5923/c.materials.201501.01
Antibacterial Activity of ZnO Powders Preparation via Sol-Gel Method
Weerachai Sangchay
Faculty of Industrial Technology, Songkhla Rajabhat University, Songkhla, Thailand
Correspondence to: Weerachai Sangchay, Faculty of Industrial Technology, Songkhla Rajabhat University, Songkhla, Thailand.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The ZnO powders were prepared by sol-gel method. The prepared powders were calined at the temperature of 300, 500 and 700°C for 1 h with the heating rate of 10°C/min. The microstructures of the fabricated powders were characterized by SEM and XRD techniques, and the results show that all samples were the agglomeration and spherical shape and reveal only the wurtzite phase. The antibacterial activity efficiency was evaluated by the inactivation of E. coli. It was observed that higher calined at the temperature gives better antibacterial activity. With the highest calined at the temperature investigated in this experiment (T700 condition) the powders show antibacterial activities of 99.00% for under UV irradiation 20 min.
Keywords: Antibacterial activity, ZnO powders, E. coil, Sol-Gel method
Cite this paper: Weerachai Sangchay, Antibacterial Activity of ZnO Powders Preparation via Sol-Gel Method, American Journal of Materials Science, Vol. 5 No. 3B, 2015, pp. 1-4. doi: 10.5923/c.materials.201501.01.
Article Outline
1. Introduction
- Zinc oxide (ZnO) is an important inorganic material, which has multiple properties, such as semiconducting properties, antibacterial activity and growth promoter [1]. It is widely applied in the field of optoelectronics [2-3], pharmaceutics [4], cosmetics [5-6], food science [7-8] and agriculture [1]. The antibacterial activity of ZnO has been widely explored [1, 9-11]. It has been documented that concentration, size and healing temperature can affect the antibacterial activity. ZnO as an inorganic antibacterial reagent is more stable than the organic reagents [1].Up to now, a number of chemical routes have been used to synthesize ZnO powders such as hydrothermal method [12-13], spray pyrolysis [14-15] and sol-gel method [16-17]. Among these methods, sol-gel shows many advantages over other techniques such as its simplicity and low equipment cost. Therefore, in this study we had concentrated the effect of calined temperature on controlling the structural and antibacterial activity of ZnO powders by sol-gel method.
2. Experimental and Details
2.1. Powders Preparation
- ZnO powders were prepared via sol-gel method [16]. Firstly, 2.1949 g of zinc acetate dehydrate (Zn(CH3COO2). 2H2O) was dissolved in 50 ml distilled water and mixture the vigorously stirred at room temperature for 15 min. Finally, 1.6 g NaOH that was dissolved in 50 ml distilled water was slowly added to the zinc precursor solution. The white precipitates were achieved and were then vigorously at room temperature for 45 min before filtering, rising with distilled water, drying at 60°C for 24 h and calcined at the temperatures at 300, 500 and 700°C in air for 1 h with a heating rate of 10°C/min.For this work the ZnO powders calcined at the temperatures at 300, 500 and 700°C were designated as T300, T500 and T700, respectively.
2.2. Powders Characterizations
- The morphology and particle size of the fabricated powders were characterized by Scanning Electron Microscope (SEM-Quanta 400). The phase composition was characterized using an X-ray diffractometer (XRD) (Phillips X’pert MPD, Cu-K). The crystallite size was calculated by the Scherer equation, Eq. 1, [18].
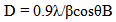 | (1) |
2.3. Antibacterial Activity Test
- The antibacterial activity of powders against the bacteria Escherichia coli (E. coli) was studied. Aliquots of 10 ml E. coli conidial suspension (105 CFU/ml) were mixed with 0.05 g of powders. The mixture was then exposed to either UV irradiation (eleven 50 W of black light lamps) for 0, 5, 10, 15 and 20 min. Then, 0.1 ml of mixture suspension was sampled and spread on Macconkey Agar plate and incubated at 37°C for 24 h. After incubation, the number of viable colonies of E. coli on each Macconkey Agar plate was observed and disinfection efficiency of each test was calculated in comparison to that of the initial or control (N/N0) [18-19]. Percentage bacterial reduction or E. coli kill percentage was calculated according to the following equation, Eq. (2) [18-19].
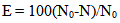 | (2) |
3. Result and Discussion
3.1. Powders Characterizations
- The surface morphology was observed with SEM. Figure 1 shows surface morphologies of ZnO powders. It was seen that for all powders, the agglomeration and spherical shape with increasing calcined temperatures at 300 to700°C.
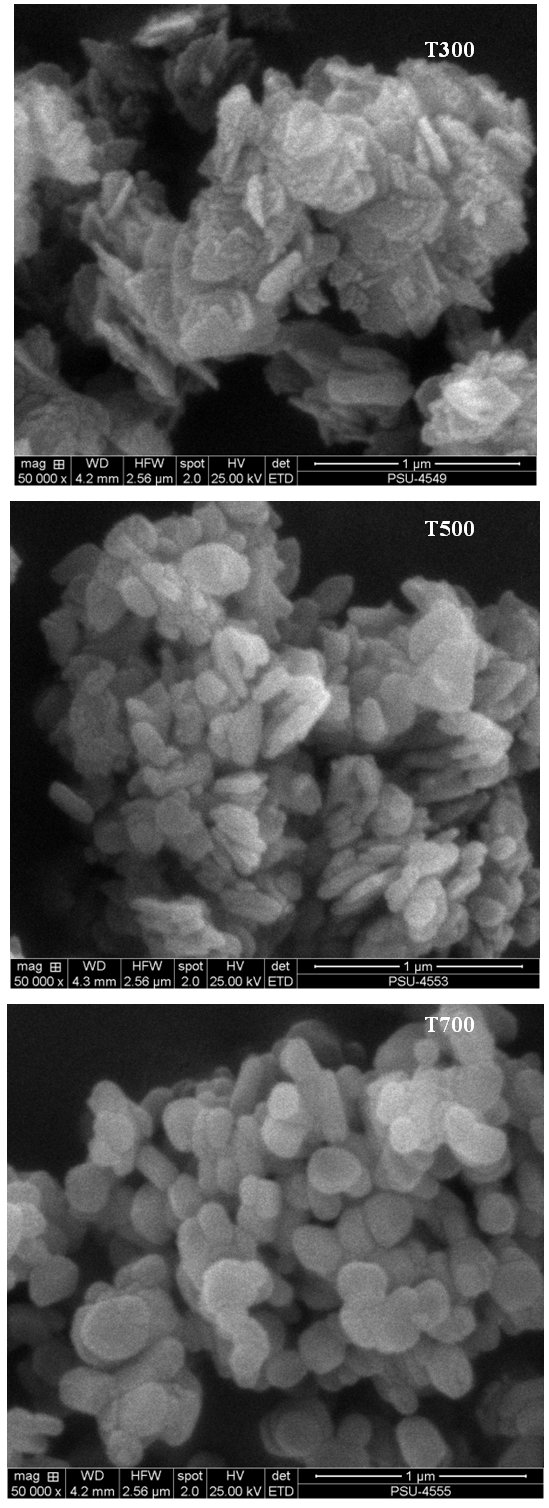 | Figure 1. SEM surface morphology images of ZnO powders calcined at the temperatures at 300, 500 and 700°C (magnification 5,000X) |
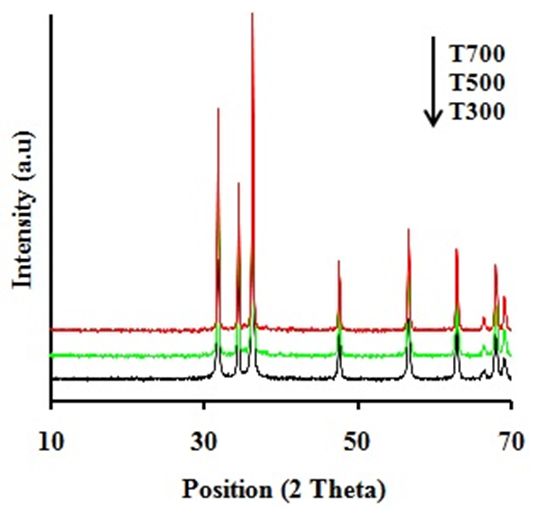 | Figure 2. XRD patterns of ZnO powders calcined at the temperatures at 300, 500 and 700°C |
3.2. Antibacterial Activity
- Figure 3 displays the E. coli survival rate (N/N0) after testing with UV illumination on ZnO powders. The result shows that the E. coli survivals decrease with UV irradiation time. It also indicates that the ZnO powders calcined at 700°C exhibit higher antibacterial activity compared to the ZnO powders calcined at 300 and 500°C, respectively.
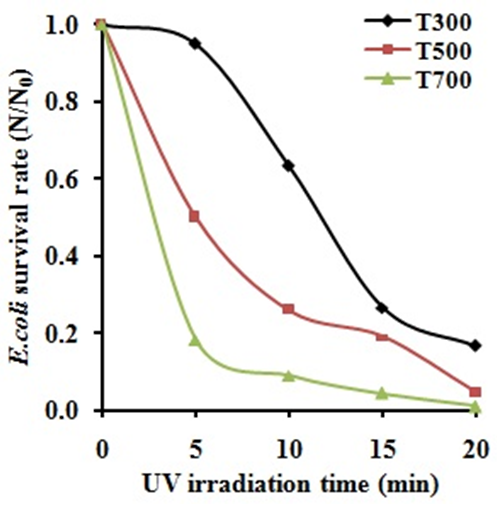 | Figure 3. The antibacterial activity of ZnO powders under UV irradiation |
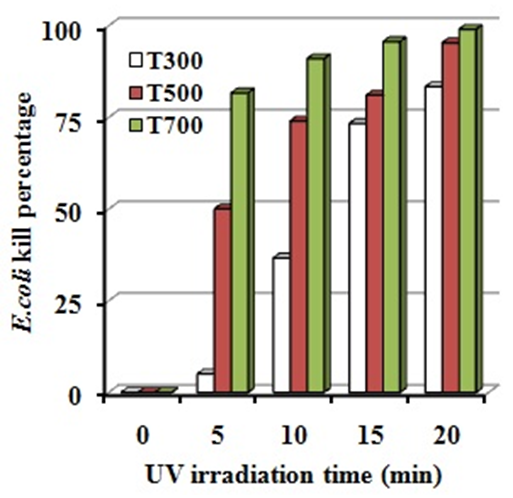 | Figure 4. E. coli kill percentage of ZnO powders under UV irradiation |
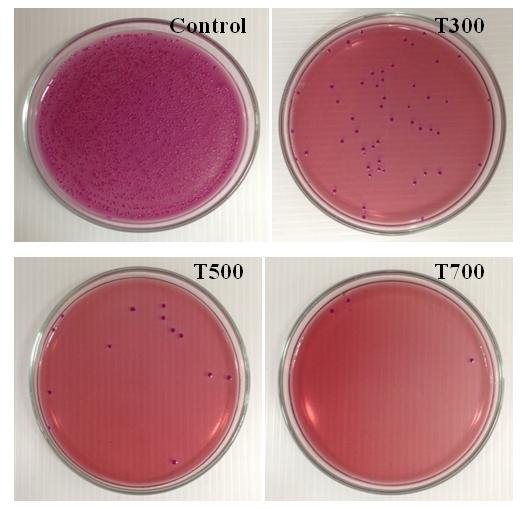 | Figure 5. Photo of viable E. coli colonies during 20 min UV irradiation of ZnO powders calcined at the temperatures at 300, 500 and 700°C compared with control condition |
4. Conclusions
- In this work, ZnO powders were fabricated by sol-gel method. The effect of calcined at the temperatures at 300, 500 and 700°C on microstructure and antibacterial activity were investigated and concluded as followings:1. ZnO powders reveal only the wurtzite phase and surface morphologies was found that for all powders, the agglomeration and spherical shape was observed and the particle size increases with increasing calcined temperatures.2. The antibacterial activities of ZnO powders increases with increasing calcined temperatures and thus, ZnO powders calcined at the temperatures at 700°C (T700) exhibits higher antibacterial activities under UV irradiation with E. coli kill percentage of 99.00% for 20 min.
ACKNOWLEDGEMENTS
- The authors would like to acknowledge Institute of Research & development, Songkhla Rajabhat University, Thailand for financial support of this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML