Nurasiah Anuar1, Chee-Ming Chan2, Angzzas Sari M. Kassim2
1Faculty of Civil and Environmental Engineering, UTHM, Parit Raja, Johor
2Faculty of Engineering Technology, UTHM, Parit Raja, Johor
Correspondence to: Chee-Ming Chan, Faculty of Engineering Technology, UTHM, Parit Raja, Johor.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Knowing the survival of Escherichia coli (E. coli) is important in evaluating the fecal contamination level in dredged marine soils. The significant factors in the survival of E. coli in dredged marine soils are important to know because marine soils can act as reservoir for bacteria. The soils physico-chemical properties are likely to affect the survival of bacteria. In fact, the relationship between soil characteristic such as type of soil, particle sizes, pH, moisture content and organic matter were related with the pattern of bacteria concentration. A study was conducted on the physico-chemical properties of marine soils samples dredged from two different dredging sites in Peninsular Malaysia. The results of the present study show that physico-chemical properties of the dredged marine soil measured varied among two dredging sites. The soils with finer fraction, high in pH, moisture content and organic matter appear to have high concentration of bacteria.
Keywords:
Escherichia Coli, Malaysia dredged marine soils, Soil physico-chemical properties
Cite this paper: Nurasiah Anuar, Chee-Ming Chan, Angzzas Sari M. Kassim, Interactions between the Survival of Escherichia coli and the Physico-Chemical Properties in Malaysia Dredged Marine Soils, Journal of Civil Engineering Research, Vol. 4 No. 3A, 2014, pp. 63-65. doi: 10.5923/c.jce.201402.10.
1. Introduction
Dredging is a necessary activity in civilization development which involves the excavation of marine soils from the bottom of waterways. The marine soils excavated are known as dredged marine soils (DMS). Million cubic meters of marine soils was dredged every year. This is due to the continuous demand for maintenance. For instant, the maintenance dredging project at Marina Melaka dredged about 120 000 cubic meter of marine soils [1]. In Malaysia, DMS are often deposited offshore, thoughtless to reuse. The marine soils could be contaminated by human activities including untreated discharge from near shore population which contains various organic and inorganic chemicals with potential risk on aquatic organism and human as well [2]. The contaminants in the dredged marine soils include oil and grease, pesticides and microbial agents. Among these contaminants, microbial agents are a major problem. Pathogens such as Escherichia coli (E. coli) can lead to severe diseases and possible death [3]. An epidemiology study revealed the most frequently used indicators of fecal contamination; E. coli was better than coliforms [4]. Instead of indicating the presence of fecal contamination, E. coli also used to indicate the presence of pathogenic microorganisms [5]. It is important to know that not all water body is the same. Thus, the marine soils physico-chemical properties and microorganisms survival will be different. Several study found that distribution of bacteria were depends on the soils physico-chemical properties. The objectives of the present study were to examine the physico-chemical properties of dredged marine soils and to study the concentration of the indicator bacteria, E. coli in the soils.
2. Materials & Methods
Field site description and sampling procedureThe dredged marine soils sample was obtained from dredging work at Marina Melaka and Tok Bali. The soils was dredged at a depth of 3.5m and packed in sampling bags and maintained at 4ºC before analysis. The sampling site were chosen as shown in Fig. 1. The sampling sites were chosen based on schedule of dredging work in East Malaysia by Jabatan Laut Malaysia.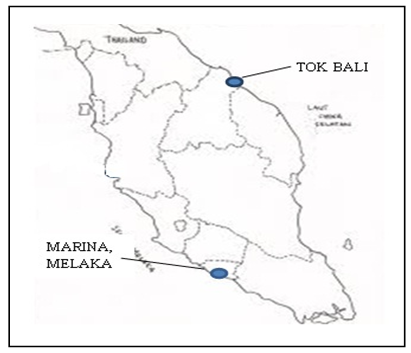 | Figure 1. Location of dredging sites |
Marine soils physico-chemical analysisThe marine soils were mixing manually to provide a homogeneity condition. The pH values of sediment were measured by mixing sediment and distilled water in 1:1 ratios. The pH value was taken after 30 minutes by inserting the probe in sediments solution [6]. The pH of samples was measured using the Eutech Instruments probe. The analysis of particle size, organic matter, and moisture content were conducted according to the British Standard 1377:1990. Bacteria enumerationSediment solution was prepared by mixing soils samples with sterilized distilled water at ratio 1:1. Once the supernatant of the sediment solution was retrieved, it was processed for isolation using Chromocult Coliform Agar (CCA). Samples were collected with sterile 1-ml pipettes and enumerated by spread plating onto Chromocult agar. All cultures plates were incubated for 24 hours at 37℃ before colony forming unit were counted [9]. The number of colonies was counted with between 30 and 300 colonies present, utilizing colony counter. The number of colonies with blue to violet were counted and recorded as the Colony Forming Unit per milliliter (CFU/ml).
3. Results and Discussions
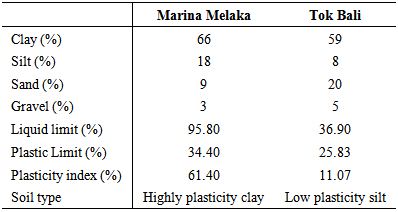
The marine soils from Marina Melaka and Tok Bali contain a mixture of clay, silt, sand and gravel in different proportions (Table 1). Overall, both soils contain high percentage of clay and low percentage of gravel. However, it appears that Marina Melaka samples has high percentage of silt with low percentage of sand while Tok Bali marine soils have low percentage of silt and high percentage of sand. Oliver [7], found that 65 % of E. coli was associated with soil particle less than 2 µm in diameter. E. coli was also observed to show greater adhere to silt and clay than sand particles [8]. Table 1. Particle size and soil type of dredged marine soils
 |
| |
|
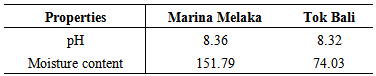
Soil structure depends on the association between soil particles (sand, silt, and clay) in which aggregates of different sizes are formed [10]. According to the Unified Soil Classification System (USCS), the fine-grained soil was classified according to the plasticity. The classification criteria are based on the relationship between Liquid Limit (LL) and Plasticity Index (PI). The relationship was determined from the plastic chart. Based on the USCS, the marine soil from Marina Melaka was placed in highly plasticity clay categories. The marine soil from Tok Bali then categorized as low plasticity silt. According to Sessitich [11], the number of bacteria was most high in the smaller size silt and clay fraction. Therefore hypothetically, DMS from Marina Melaka is expected to have high numbers of E. coli. pH value of the DMS from both sites was found to be in the alkaline range. Yet, the pH value has shown slightly changes in both dredging areas with the value 8.36 and 8.32 (Table 2). According to Rousk [12], bacteria populations in soil decline at low pH level and were growing fastest at pH above 7. Hypothetically, the pH recorded in this present study was suitable for bacteria growth. The moisture content from the Marina Melaka and Tok Bali dredging sites were from 151.79 % and 74.03 %. The Low moisture content may limit microbial activity by lowering intracellular water potential [13]. High number of bacteria was determined in soils with high moisture content [14] suggesting, that Marina Melaka DMS may have a high number of bacteria than Tok Bali samples. Table 2. pH value and moisture content in marine soils
 |
| |
|
The result shows that organic matter was high in Marina Melaka sample (Table 4.3). Dafini [15] discovered the relation between the textures of sediment with organic matter content. It was reported that higher organic matters was found associated with the finer fraction of sediment. The same trend was also observed in this study where lower organic matter was found in Tok Bali sample which has higher silt particles than the Marina Melaka sample.Table 3. Organic matter in marine soils
 |
| |
|
Soils appear to serve as a hospitable environment for bacteria survival due to the availability of organic matter [16]. Soil microorganisms play a significant role in the decomposition of organic matter. It was formed by metabolic action of the organisms [17]. In order to obtain carbon and energy from the sediment organic matter, the bacteria has to degrade organic macro-molecules such as proteins and nucleic acid to simpler substrates. Thus, organic matter provides food for microorganisms in the soil through decay or decomposition of plant and animal residue [18]. Therefore, the high organic matter in marine soils could be the result of high concentration of bacteria. Data obtained on the bacteria concentration in marine soils for both locations is shown in Table 4. The higher numbers of E. coli was found in Marina Melaka samples. Such differences in the bacteria concentration could be related with the varieties in physico-chemical characteristics among the soils samples. The result of the present study was corresponded with the findings of the other study that reported the influence of soils particle size, pH, moisture content as well as organic matter content with the bacteria concentration level [19]. The soils with finer fraction, high in pH, moisture content and organic matter appear to have high concentration of bacteria. Table 4. Concentration of E. coli in dredged marine soils
 |
| |
|
ACKNOWLEDGEMENTS
The research for this project was financially supported by Ministry of Higher Education under Research Acculturation Collaborative Effort (RACE) grant. We would like to thank Universiti Sains Malaysia (USM) for collaboration and helpful input. We also would like to thank Jabatan Laut Malaysia and Universiti Tun Hussein Onn Malaysia (UTHM) for the technical support.
References
| [1] | Jabatan Laut Malaysia, 2013. |
| [2] | P. Vervaeke, S. Luyssaert, J. Mertens, E. Meers, F.M.G. Tack, & N. Lust, (2003) Phytoremediation propects of willow stand on contaminated sediment: a field trial. Environmental Pollution, 126 (2003), pp. 275 – 282. |
| [3] | C. Mulligan, M. Fukue, & Y. Sato. Sediments Contamination and Sustainable Remediation. New York: IWA Publishing. 2010. |
| [4] | L. Fewtrell, & J. Batram, (2001) Water Quality: Guidelines, standards and health. World Health Organization Water Series, pp. 20-21. |
| [5] | R.A. Blaustein, Y. Pachepsky, R.L. Hill, D.R. Shelton, & G. Whelan. (2013). Escherichia coli survival in waters: Temperature dependence. Water research, 47, pp. 569-578. |
| [6] | F.L. Singleton, R. Attwell, S. Jangi, and R.R. Colwell, “Effect of temperature and salinity on Vibrio Chlorae growth,” Applied and Environmental Microbiology, Vol. 44, pp. 1047- 1058, Nov 1982. |
| [7] | D.M. Oliver, C.D. Clegg, A.L. Heathwaite & P.M. Haygarth, (2007). Preferential attachment of Escherichia coli to different particle size fraction of an agricultural grassland soil. Water, Air, Soil Pollution, 185, pp. 369-375. |
| [8] | A.K. Guber, Y.A. Pachepsky, D.R. Shelton, & O. Yu, (2007). Effect of bovine manure on fecal coliform attachment to soil and soil particles of different sizes, Applied and Environmental Microbiology, 73, pp. 3363-3370. |
| [9] | M.A. Arshad, P. Bakar, and M. Samingin, “Integration of fisheries into coastal area management: Case of Semerak Lagoon”. |
| [10] | Tisdall J.M. & Oades J.M. (1982). Organic matter and water stable aggregates in soils, Journal of Soil Science, 33, pp. 141-163. |
| [11] | Sessitch A., Weilharter A., Gerzabek M.H., Kirchmann H. & Kandeler E. (2001). Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment, Applied and Environmental Microbiology, 67(9), pp. 4215-4224. |
| [12] | Rousk J., Brookes P.C. & Baath E. (2009). Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Applied and Environmental Microbiology, 75(6), pp. 1589-1596. |
| [13] | Stark J.M. & Firestone M.K. (1995). Mechanisms for soil moisture effects on activity of nitrifying bacteria. Applied and Environmental Microbiology. 61(1), pp. 218-221. |
| [14] | Stres B., Danevčič T., Pal L., Fuka M.M., Resman L., Leskovec S., Hacin J., Stopar D., Mahne I. & Mandic-Mulec I. (2008). Influence of temperature and soil water content on bacterial, archaeal and denitrifying microbial communities in drained fen grassland soil microcosms. Federation of European Microbiological Societies, 66, pp. 110-122. |
| [15] | Dafini M., Ramya K.D., Jimly C.J. & Rosamma P. (2013). Heterotrophic bacterial and fungal diversity in the inner shelf sediments of central west coast of India. Advance in Applied Science Research, 4(4), pp. 490 – 500. |
| [16] | Alam M.W. & Zafar M. (2013). Spatial and temporal variation of Escherichia coli in water and soil with relation to water parameters at the estuary of Karnafuly River, Bangladesh. Journal of Bacteriology Research, 5(1), pp. 1-8. |
| [17] | Mirsal I.A.. Soil Pollution: Origin, Monitoring & Remediation. 2nd Ed. German: Springer. 2008. |
| [18] | Arnosti C., Jorgensen B.B., Sagemann J. & Thamdrup B. (1998). Temperature dependence of microbial degradation of organic matter in marine sediments: polysaccharide hydrolysis, oxygen consumption, and sulfate reduction. Marine ecology progress series, 165, pp. 59-70. |
| [19] | Hossain M.Z., Aziz C.B. & Saha M.L. (2012). Relationship between soil physico-chemical properties and Total Viable bacteria counts in Sunderban Mangrove Forests, Bangladesh, Journal of Biological Science. 21(2), pp. 169-175. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


