-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Civil Engineering Research
p-ISSN: 2163-2316 e-ISSN: 2163-2340
2014; 4(2A): 51-54
doi:10.5923/c.jce.201401.10
Soil Bacteria for the Strength Enhancement of Cement Mortar
Sujatha S., Sarayu K., Annaselvi M., Ramachandra Murthy A., Ramesh Kumar V., Nagesh R. Iyer
CSIR-Structural Engineering Research Centre, CSIR Road, Taramani, Chennai - 600 113
Correspondence to: Sarayu K., CSIR-Structural Engineering Research Centre, CSIR Road, Taramani, Chennai - 600 113.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Concrete is the widely used construction material worldwide. But the production of the cement used in it contribute to almost 7% of the global anthropogenic CO2 emission, which has adverse environmental impact. The cracking phenomenon, pore structure, permeability hampers the materials structure and integrity. Bacteria is able to transform soluble organic nutrients into insoluble inorganic calcite crystals, which when applied to concrete seals the cracks. Present study has clearly evidenced the precipitation of calcium carbonate by the bacteria with the optical micrographs. The 28th day compressive strength of the bacterial cement mortar is also found to increase up to 18% when cured with water containing urea and up to 12% when cure with water.
Keywords: Bacteria, Calcium carbonate, Cement mortar, Strengthening
Cite this paper: Sujatha S., Sarayu K., Annaselvi M., Ramachandra Murthy A., Ramesh Kumar V., Nagesh R. Iyer, Soil Bacteria for the Strength Enhancement of Cement Mortar, Journal of Civil Engineering Research, Vol. 4 No. 2A, 2014, pp. 51-54. doi: 10.5923/c.jce.201401.10.
Article Outline
1. Introduction
- Biomineralization of calcium carbonates, that is, the biologically controlled or induced mineralization of CaCO3, is an important and ubiquitous process resulting in biominerals produced by a range of taxa, from bacteria and archaea to eukarya. [2] Calcium carbonate precipitation by a large number of bacterial genera has been observed in a range of natural environments, including soils, caves, lakes and sea water as well as in the laboratory. Bacterial mineralization of calcium carbonates has wide range of applications [7]. Biomineralisation process forms precipitation and fills the pores that enhance the mechanical properties in concrete materials such as strength and durability. It is reported that some specific microorganisms manage to make a transformation for most elements in the periodic table. For example, Shewanella sp. is able to produce calcium carbonate (calcite) that binds to the wall of the bacteria [1].The most serious problem of concrete is that it can easily crack when under tension. If these cracks expand to large cracks, they will lead to corrosion of the steel reinforcement, which not only results in an unattractive appearance, but also jeopardizes the structure’s mechanical qualities. It is possible to create concrete that has self- healing capacities by adding calcite-precipitating bacteria [3].Researchers are developing similar self-healing concrete in which bacteria mediate the production of minerals that rapidly seal freshly formed cracks. This process that concomitantly decreases concrete permeability, and thus better protects embedded steel reinforcement from corrosion. [4]. Initial results have shown that the addition of specific organic mineral precursor compounds plus spore-forming alkaliphilic bacteria as self-healing agents produces up to 100-μm sized calcite particles which can potentially seal micro- to even larger sized cracks. Further development of this bio-concrete with significantly increased self-healing capacities could represent a new type of durable and sustainable concrete with a wide range of potential applications [5].
1.1. Objective and Scope
- This study deals with a vital strain named as IsAH-1 isolated from an ant hill which is used to evaluate the improved mechanical properties of the cement mortar. This study clearly brings out the calcareous nature of the bacterium isolated and its efficiency in improving the mechanical properties of the cement mortar.
2. Material and Methods
2.1. Isolation of Bacteria
- Ant hill samples were collected when it was freshly constructed by the termites. Then the sample was serially diluted and plated to obtain the different types of bacterial colonies. The isolated colonies were further purified by streaking it in to a Nutrient Agar (HiMedia, India) slant and were incubated overnight. These were further maintained as glycerol stocks.
2.2. Identification of Calcareous Organisms
- The isolated strains were inoculated to Christenson's Urea agar (HiMedia, India) to know their urease activity as urease enzyme is responsible for the calcium carbonate formation and precipitation by the bacteria. It was also streaked in a calcium carbonate precipitation medium (Media composition per litre- urea 20g, sodium carbonate 2.12g, ammonium chloride 10g, nutrient broth 3g, calcium chloride dihydrate 25g at pH 7.5 to 8) that can enhance the calcium carbonate precipitation through the other metabolic pathways.
2.3. Preparation of Culture
- The culture for casting was prepared by growing the isolate IsAH-1 in 4.5L of Luria Bertani broth (HiMedia, India) with 0.5% of cement, 0.5% of broken concrete powder and 0.5% of urea to obtain a cell concentration of 4.8x107 CFU/ml. Then the bacterial cells were harvested by centrifugation. The pellet of the centrifuged culture was mixed with the water required for casting and was used immediately for casting.
2.4. Bacterial Cement Mortar
- The cement mortar of 70.6mm/70.6mm/70.6mm was prepared using 53 grade ordinary portland cement of IS 12269-1987 standards and grade 2 sand of IS 383-1970 for the study. The water cement ratio was maintained as 0.4 based on the standard consistency tests carried out. Control and Test-1 specimens were cured with water and Test-2 specimens were cured with water containing 1g urea/L. The cubes after curing with water was tested for compressive test on the 3rd, 7th and the 28th days of curing.
2.5. Characterization
- The calcium carbonate crystals secreted by the bacteria was characterized for its structural morphology using the optical microscopy. Then the calcium carbonate precipitation in the bacterial cement mortar cubes were characterized based on the XRD results obtained from the 3rd, 7th and the 28th days of curing.
3. Results and Discussion
3.1. Precipitation of Calcium Ccarbonate
- The isolate IsAH-1 was found to produce urease enzyme turning the Christenson's Urea agar slant pink due to the pH sift to alkaline indicating the production of ammonia in the medium "Figure 1".
 | Figure 1. Urease production by the isolate |
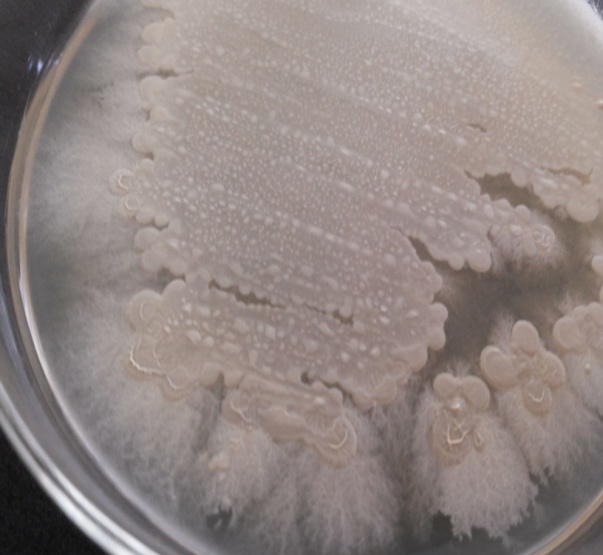 | Figure 2. Observation of the calcium carbonate precipitation |
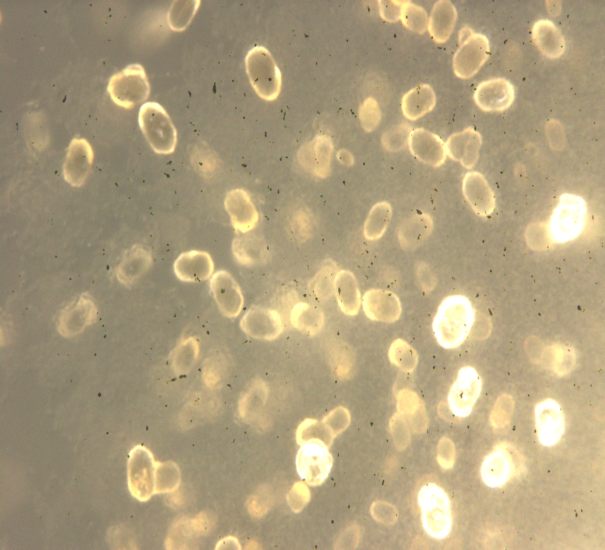 | Figure 3. Optical micrographs of the calcium carbonate crystals formed by the isolate IsAH-1 |
3.2. Evaluation of Improved Mechanical Properties and Calcite Precipitation
- The control specimens achieved a compressive strength of 41.0 MPa which is relatively closer to the grade of the cement used ie. 53 MPa in 28 days. The control mortar cubes attained about 48% of the 28th day strength in 3rd day of curing and about 65% of 28th day strength in 7th day of curing in water "Figure 4".
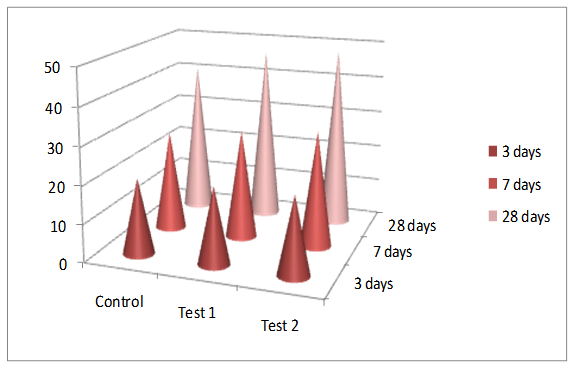 | Figure 4. Evaluation of compressive strength 1: Control; 2: Test 1, Test 2 |
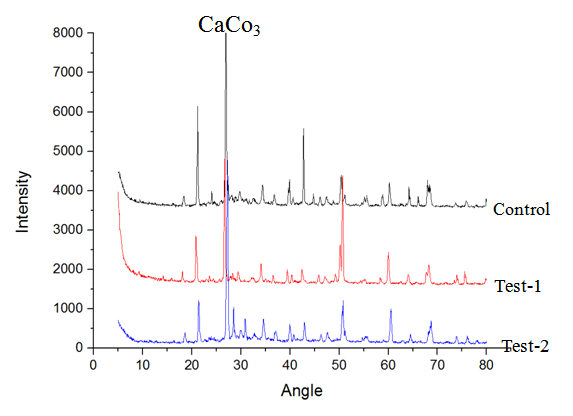 | Figure 5. XRD of the mortar specimens |
4. Conclusions
- As the bacteria used for the study is a urease positive strain it has utilized the urea present in the curing medium as its nitrogenous source. This has induced the precipitation of calcium carbonate with the well know ureolytic pathway. This property of the bacteria has contributed to the increase in the 28th day compressive strength of the Test-2 specimens to about 18% compared to the control specimens. Whereas, the Test-1 specimens that were cured with water has shown only a 12% increase in the compressive strength compared to that of the control specimens.
ACKNOWLEDGEMENTS
- The authors are thankful to the Director, CSIR- Structural Engineering Research Centre, Chennai for allowing us to publish this work. Part of the research work has been carried out with equipment of CSIR innovation Complex for which authors would like to thank Director, CSIR-SERC.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML