-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2015; 5(3A): 48-51
doi:10.5923/c.chemistry.201501.07
4-Hydroxycoumarins as New Fluorescent Compounds: Synthesis and Characterization
Yasameen K. Al-Majedy1, 2, Ahmed A. Al-Amiery1, 2, Abdul Amir H. Kadhum1, Abu Bakar Mohamad1
1Department of Chemical and Process Engineering, Universiti Kebangsaan Malaysia (UKM)
2Chemical Division, Department of Applied Science, University of Technology Baghdad, Iraq
Correspondence to: Yasameen K. Al-Majedy, Department of Chemical and Process Engineering, Universiti Kebangsaan Malaysia (UKM).
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
New 4-hydroxycoumarins compounds were designed, synthesized and characterized as fluorescent compounds. Absorption and fluorescence spectra of these derivatives have been investigated in alcoholic media. The substituting groups with varying electron donating ability exhibits fluorescence at a longer wavelength i.e. 420-460 nm in ethanol in compound (1-5). Compounds 1-5 exhibited high quantum yield in ethanol. The fluorescence intensities of compounds 2 and 5 were extremely strong. Compounds 2 and 5 exhibited high fluorescent quantum yields in solution compared to compounds 1,3,4.
Keywords: Fluorescence, 4-hydroxy-coumarin, Emission, Synthesis
Cite this paper: Yasameen K. Al-Majedy, Ahmed A. Al-Amiery, Abdul Amir H. Kadhum, Abu Bakar Mohamad, 4-Hydroxycoumarins as New Fluorescent Compounds: Synthesis and Characterization, American Journal of Chemistry, Vol. 5 No. 3A, 2015, pp. 48-51. doi: 10.5923/c.chemistry.201501.07.
Article Outline
1. Introduction
- Fluorescent compounds play an important role in solid state lighting, solar energy conversion and as biomarkers in the life sciences [1-6]. As an important group of organic heterocycles, coumarin derivatives have been found to possess versatile pharmacological/biological activities, which can display anticancer [7, 8], anti-HIV [9], antiviral [10], antimicrobial [11], anti-inflammatory [12, 13], and antioxidant [14] activities, 4-Hydroxycoumarin and its derivatives have been effectively used as anticoagulants for the treatment of disorders in which there is excessive or undesirable clotting, such as thrombophlebitis [15], pulmonary embolism [16], and certain cardiac conditions [17]. Moreover, their unique photochemical and photophysical properties make them useful in a variety of optical application such as in optical brighteners, laser dyes, nonlinear optical chromophores, solar collector systems and organic lightemitting diodes (LED). Coumarin fluorescent probes or labels have extensive and diverse applications, as they exhibit extended spectral range and high emission quantum yields [18]. Coumarin-based fluorescent chemodosimeter with salicylaldehyde functionality were used as a binding site for selective detection of cyanide anions over other anions in water at biological pH [19], Shihai Zhou et al., [20] have studied 3-(2-benzothiiazolyl) coumarins for their fluorescent properties in which the coumarin acts as a donor while the benzothiazole moiety acts an acceptor. P Moeckli et al., [21] introduced some new red fluorescent coumarin molecules by keeping electron donating group on 7th position of the coumarin ring and withdrawing group on 3-position. The oxygen atom at the 3-carbonyl group act as a hydrogen bond acceptor and electron donating group on 7th position enhance the fluorescence emission [22] Chiyomi Murata et al., [23] examined coumarin derivatives with both electron donating groups at the 6- and 7-posotions and an electron-withdrawing group at the 3-position, which develop intense fluorescence.In this work, we present simple novel fluorescents coumarins synthesis and characterization by the means of NMR, FT-IR techniques in addition of CHN analysis. We utilize the unique advantage of simple coumarins as eco-friendly fluorescents compounds. Moreover, the approach of synthesized these fluorescent compounds are demonstrated in scheme 1.
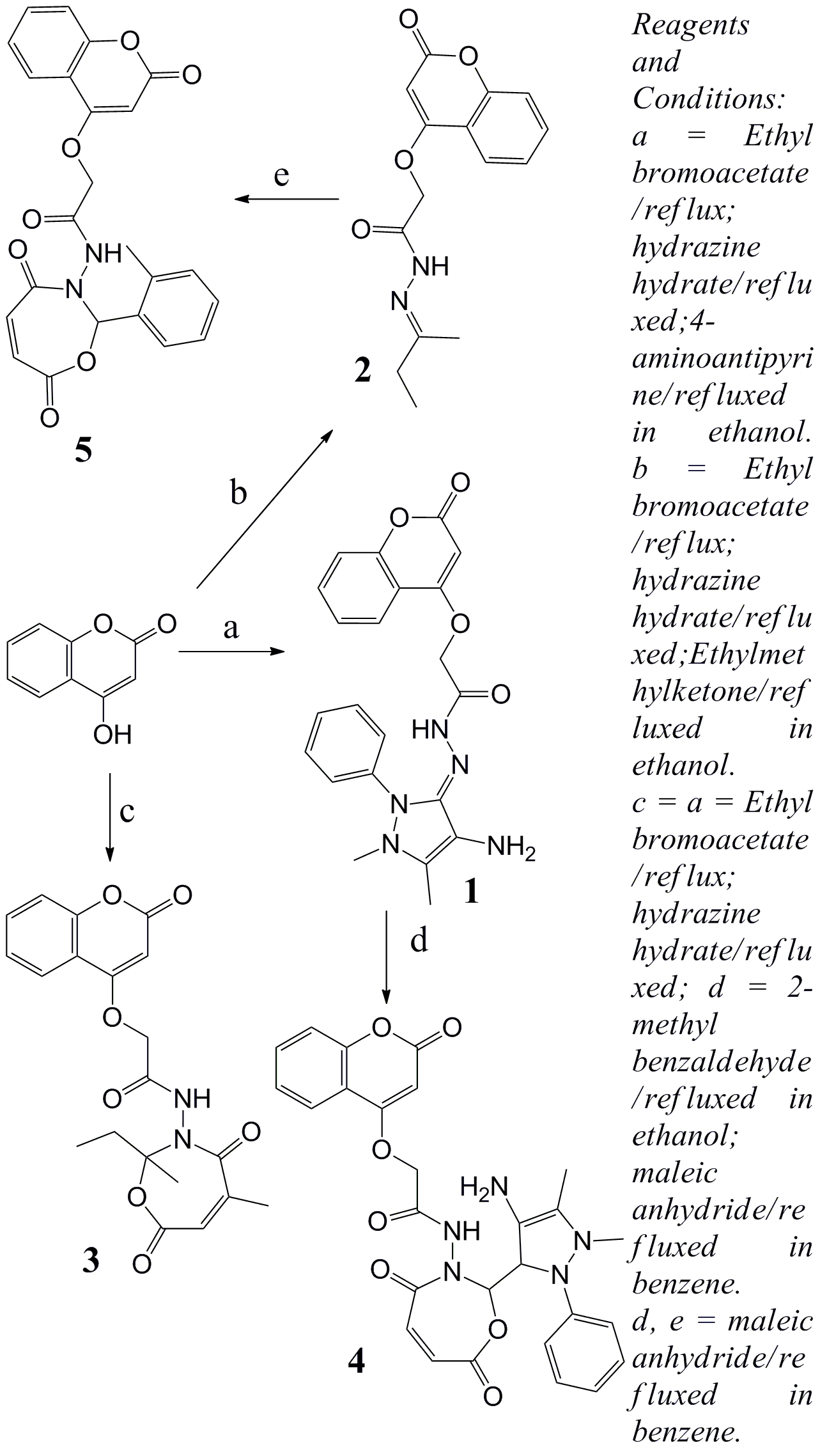 | Scheme 1. Reaction sequences of the synthesized compounds |
2. Experimental Section
2.1. Chemistry
- All synthesized compounds were published by the Al-Amiery et al. [24].
2.2. Fluoresce
- The fluorescence spectra were recorded on F-8500 spectrofluorometer ethanol at a concentration of 1mg mL–1. The fluorescence spectra were recorded using excitation into the maximum of the longest wavelength absorption band program. The fluorescence of the solution was measured in a 1cm3 cuvette in the right angle arrangement. The quantum yield of the coumarin derivatives were determined using Rhodamine B as the standard in the respective medium. The fluorescence spectra were taken by the excitation into the maximum of the longest wavelength absorption band. Calculation of the quantum yieldΦx = Φs[As/Ax][Rs/Rx][D/Ds]Φ = Fluorescence quantum yieldSubscritps x and s denotes test and standard respectivelyR = refractive index of the solventD = area under the corrected, extrapolated emission spectra [25, 26].
3. Result and Discussion
3.1. Fluorescence Properties
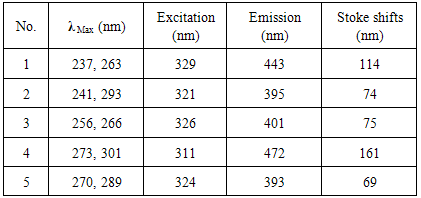
- The studies of fluorescence were performed in ethanol at a concentration of 1.0×10-5 mol/L, and fluorescence spectra were obtained at their respective maximum excitation wavelength Coumarin derivatives substituted an electron-donating group were known to exhibit strong fluorescence. The fluorescence quantum yields (Table 1) of compounds (1-5) were determined via comparison method. The electron donating substituent i.e.,( N-NH ) group 1-5 exhibited longer emission wavelength ranging from 393 to 472 nm [27], where benzocoumarin part served as strong donor [28-31] to constitute a strong push-pull system to exhibit high fluorescent intensities.
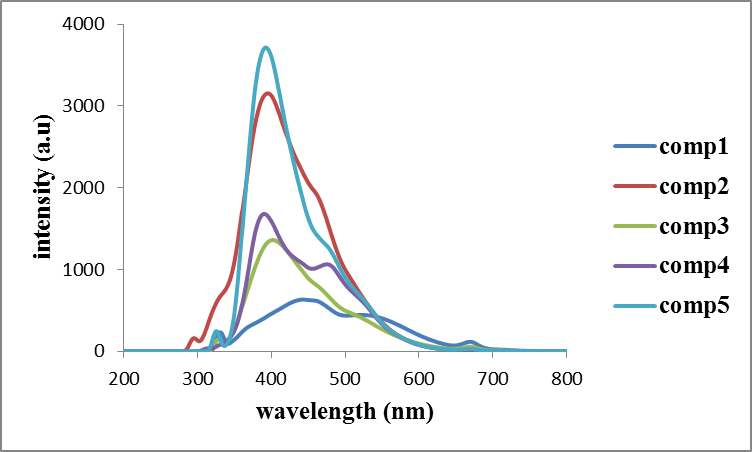 | Figure 1. Excitation spectra of compounds (1-5) |
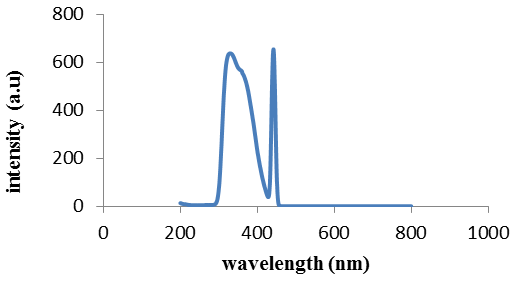 | Figure 2. Fluorescence emission of compound (1) |
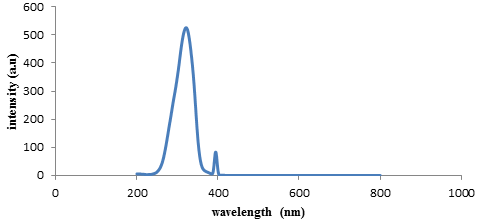 | Figure 3. Fluorescence emission of compound (2) |
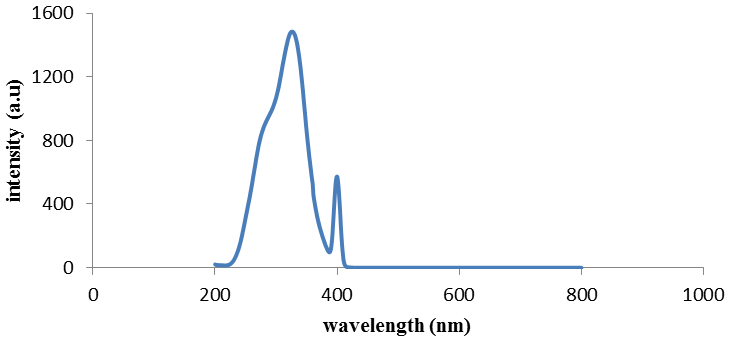 | Figure 4. Fluorescence emission of compound (3) |
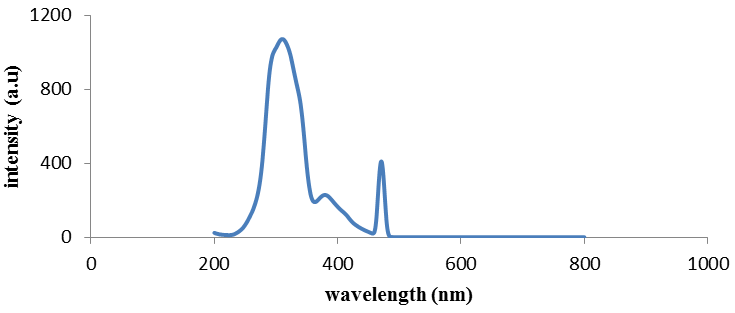 | Figure 5. Fluorescence emission of compound (4) |
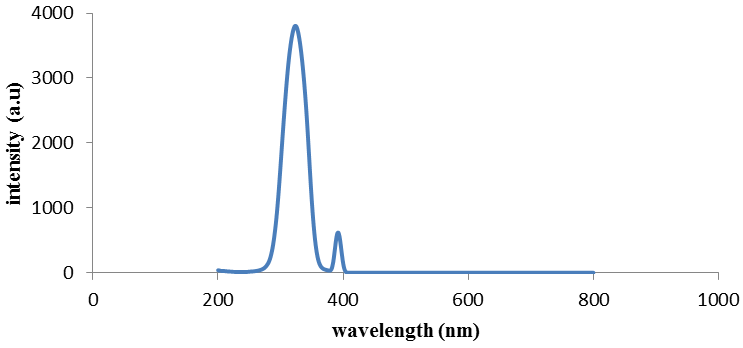 | Figure 6. Fluorescence emission of compound (5) |
|
4. Conclusions
- A series of new cyclic compounds based 4-hydroxycoumarin were successfully synthesized and structures were confirmed by spectral analysis performed by IR, NMR and elemental analysis. The fluorescence properties of all the synthesized compounds were studied in ethanol. The results obtained were interesting that the compounds (1-5) shows fluorescent in ethanol with high quantum yield and show high activity.
ACKNOWLEDGEMENTS
- This study was supported by the University of Technology, Baghdad, Iraq, and Universiti Kebangsaan Malaysia under the DIP-2012-02 Grant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML