-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2015; 5(3A): 13-23
doi:10.5923/c.chemistry.201501.03
Overview of Biohydrogen Production Technologies and Application in Fuel Cell
Rahman S. N. A.1, Masdar M. S.2, Rosli M. I.3, Majlan E. H.1, Husaini T.1
1Fuel Cell Institute, Universiti Kebangsaan Malaysia
2Research Centre for Sustainable Process Technology (CESPRO), Faculty of Engineering & Built Environment, Universiti Kebangsaaan Malaysia
3Department of Chemical and Process Engineering, Faculty of Engineering & Built Environment, Universiti Kebangsaan Malaysia
Correspondence to: Masdar M. S., Research Centre for Sustainable Process Technology (CESPRO), Faculty of Engineering & Built Environment, Universiti Kebangsaaan Malaysia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Hydrogen gas is clean and efficient energy carrier. Currently, hydrogen is produce by carbon-based non-renewable source such as natural gases, coal, heavy oil and naptha as well as from electrolysis of water. However, the productions of hydrogen from fossil fuels generate greenhouse gases and require high energy. Thus, it is important to develop alternative hydrogen production routes that are renewable such as biohydrogen. In this paper, a review of biohydrogen production technologies including the mechanism and bioreactor system, and the hydrogen purification system is focused and discussed for the application of polymer electrolyte membrane fuel cell (PEMFC). Besides that, the bioreactor sizes that require generating hydrogen to power the PEMFC stack is calculated and compared in order to determine the potential application of hydrogen production through the biological pathway.
Keywords: Biohydrogen, Purification, Membrane separation, PEMFC, Bioreactors
Cite this paper: Rahman S. N. A., Masdar M. S., Rosli M. I., Majlan E. H., Husaini T., Overview of Biohydrogen Production Technologies and Application in Fuel Cell, American Journal of Chemistry, Vol. 5 No. 3A, 2015, pp. 13-23. doi: 10.5923/c.chemistry.201501.03.
Article Outline
1. Introduction
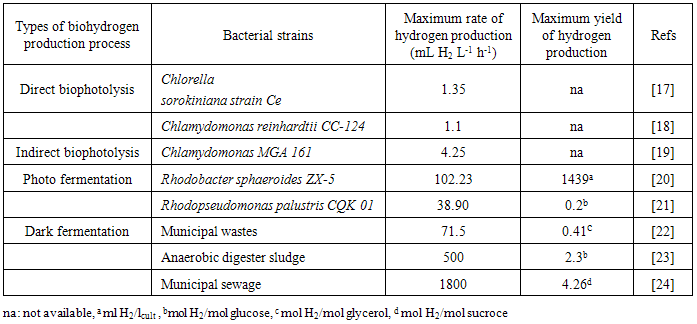
- Hydrogen has highest energy content per unit weight of any known fuel (142kJ/g) which is 2.75 times more energy density than other existing biofuels [1]. Besides that, the combustion of hydrogen gas to generate energy is environment friendly as combustion product only water vapours. Currently, hydrogen is producing from compound that containing hydrogen which natural gases, heavy oil and naphtha, coal, electrolysis of water and biomass. However, the carbon-based non-renewable source from fossil not able to sustain energy for the long term future as they currently depleted and also require high thermal energy, emit greenhouse gases to atmospheres which the disadvantage in environment view [2]. Therefore, generate hydrogen from renewable source such as biohydrogen production processes is important in order to make hydrogen as a possible clean energy carrier.Biological hydrogen (biohydrogen) production processes is a hydrogen production technology that utilise renewable energy resources by using microorganisms. The process is operating at ambient temperature and atmospheric pressure, and use less energy. These biohydrogen production technologies are possible candidate for sustain the world power energy supply, potential to replace fossil fuels and do not discharge greenhouse gases such as carbon dioxide. The biohydrogen is generating by three differents mechanism which can be categorised as light dependent (direct or indirect biophotolysis and photo fermentation) and independent (dark fermentation) [3, 4]. The best energy utilisation from hydrogen is in fuel cell application. The hydrogen gas generated from conventional process is commercially use as fuel in fuel cell system to generate electricity. However, the hydrogen produces from biohydrogen production technology for application in fuel cell is not yet commercial and the study about the application is also limited. This is because of some technical challenges of biohydrogen production technologies is need to be settle out with respect to using the hydrogen in fuel cell application. One of challenges is the hydrogen from biohydrogen production process must follow the particular fuel requirement of different fuel cell [5]. Therefore, it is important to study about the potential hydrogen separation and purification system from biohydrogen production process. Besides that, it is crucial to find a potential pathway to generate hydrogen from biohydrogen production which could bring a new step in the progress of fuel cell technologies.Therefore, this review is focused on the comparison of hydrogen production rate, advantages and disadvantages from those three mechanisms of biohydrogen production process. Other than that, the hydrogen separation and purification from bioreactor is focused and will be discussed on the membrane gas technology.In order to determine the potential application of hydrogen from biohydrogen production process in fuel cell system, polymer electrolyte membrane fuel cell (PEMFC) is chosen as it seems promising fuel cell for various applications such as stationary and portable fuel cell power generator. The comparison is done based on the calculated bioreactor size that required producing enough hydrogen from biohydrogen production process for power the PEMFC at 5W to 250kW which represent particular fuel cell application i.e. 5W-5kW represent portable power generator, 500W-100kW is stationary power generator, 5-250kW is demonstrated transportation application. The biohydrogen production process which potentially to commercial is determine based on the capability of the hydrogen produce to supply the PEMFC stack for 24 h in a continuous basis. Despite of various challenges and technical barrier to be handle and solve in order to generate hydrogen for large scale, however the purpose of this review is to give basis evaluation of fuel cell potential application from hydrogen generate from biohydrogen production process.
2. Biological Mechanism and Bioreactor for Hydrogen Production
2.1. Biophotolysis
- Photoautotrophic organism such as microalgae and cyanobacteria are capable to generate hydrogen and carbon dioxide in biophotolysis process. They utilize the light as energy source and carbon dioxide as carbon source to split water into hydrogen [6]. They produce biohydrogen by direct photolysis of water under the anaerobic conditions (1) [7]. There are two types of biophotolysis which are direct biophotolysis and indirect biophotolysis, as discussed next.
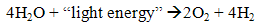 | (1) |
2.1.1. Direct Biophotolysis
- Hydrogen production by direct biophotolysis process is used solar energy and photosynthetic system of algae to convert water into chemical energy which subdivided into one-stage or two-stages. In direct biophotolysis, the light absorbed by photosystem I (PSI) and photosystem II (PSII) which use in hydrogen production and energy to transport electrons linearly to ferredoxin. The light absorbed by PSII is required for oxidation of water into electrons, protons (H+) and oxygen molecules (2). These electrons then transfer through the electron transport chain using the light energy absorbed in PSI to ferredoxin to the hydrogenase enzyme without involving intermediates CO2 fixation. These enzymes are then catalyst for recombination process of proton and electrons to evolve the hydrogen gas (3). This mechanism is also refers as one-stage direct biophotolysis where the biohydrogen is produced directly from water and solar energy via algae photosynthesis system.
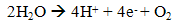 | (2) |
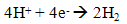 | (3) |
2.1.2. Indirect Biophotolysis
- Indirect biophotolysis is the production of hydrogen from water via microalgae and cyanobacteria photosynthetic system to convert solar energy into chemical energy which is hydrogen by two steps in series. The first step is the biomass production through photosynthetic system (4) and follow by second step which utilise the biomass rich-carbohydrate for hydrogen-producing fermentation (5).
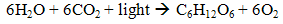 | (4) |
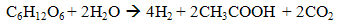 | (5) |
2.2. Photo Fermentation
- The hydrogen produce in photo fermentation is by photosynthetic organisms, in which using photosynthetic bacteria with additional of light. These photosynthetic bacteria are lack of Photosystem II (PSII) and perform the photosynthesis with Photosystem I (PSI) for hydrogen production. The process still requires light as source energy likely to biophotolysis, but the photosynthetic bacteria in this process not powerful enough to split water such in biophotolysis, so the process utilise organic acids, like acetic acid as electron donor to generate hydrogen (6).
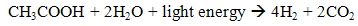 | (6) |
2.3. Dark Fermentation
- Dark fermentation is produce hydrogen via heterotrophic mechanism in which use carbohydrates like glucose as energy and carbon source and allocate the balance of energy in hydrogen and fermentation related products and not requiring light energy source which is the limitation in light dependent process, biophotolysis and photo fermentation. The same anaerobic method used in photo fermentation is also implementing in dark fermentation because this method eliminate the issue of sensitivity of hydrogenase to oxygen and give high hydrogen production. Other than carbohydrates, the process can use variety carbon source including wastewater enriched with carbohydrates, organic compound, polymer (i.e., starch, cellulose), and algae biomass (i.e., macroalgae and microalgae) which give advantages on utilization the low value waste [11]. Hydrogen production in dark fermentation is very complex process which involving various bacteria by a series of biochemical reactions. In the early stage of dark fermentation, carbohydrate, mainly glucose which is the preferred carbon source for dark fermentation process is break down into pyruvate and NADH which is called as glycolysis (7) [12].
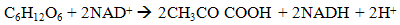 | (7) |
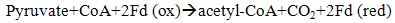 | (8) |
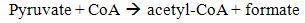 | (9) |
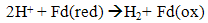 | (10) |
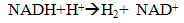 | (11) |
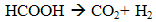 | (12) |
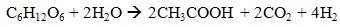 | (13) |
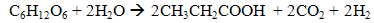 | (14) |
|
3. Purification of Biohydrogen Gas
- Hydrogen that produced during dark fermentation process is required to remove continuously for the potential continuous mode of the operation and eliminate the hydrogen accumulation that reduced hydrogen production rate. High hydrogen concentration in the bioreactor appears to inhibit the hydrogen production and more by-products such as ethanol, butanol, lactate, and acetone is generating [25]. Thus, separation and purification of biohydrogen is essential for the hydrogen utilization and also for sustained the biohydrogen production process.Many technologies have been applied for hydrogen separation such as pressure swing adsorption, solvent adsorption, cryogenic recovery and membrane. The separation and selective purification of hydrogen via membrane technology appear to be promising to replace the conventional hydrogen separation system. There are many types of membranes are existing to enrich hydrogen which can be categorised as organic or inorganic membrane and can be subdivided into porous or non-porousmembrane. The catalytic (de)hydrogenation reaction has been applied for hydrogen separation by using catalytic inorganic membrane reactors which is operated at high temperature [25]. Thus, non-porous metallic (e.g palladium) membrane is used because of their high thermostable membranes and selectivity to hydrogen. However, this type of membrane very expensive (palladium cost), less commercially available and operate at elevated temperature is impratical for biohydrogen separation as the operational condition of biohydrogen production to nearly the ambient conditions. Organic (polymer) has been tested for the separation of H2/CO2 mixture shows the promising results [26]. Non-porous polymeric membrane (NPPM) is performing separation based on transport mechanism known as solution diffusion. Generally, these type of membranes is operated at nearly same the conditions (ambient temperature and pressure) of biohydrogen production process as well as low cost, make it have a potential technology for biohydrogen separation. This type of membrane is use for gas separation in a device called as membrane contactor (MC). In another polymeric membrane, porous type is uses as supporting material in which incorporate with ionic liquids (ILs) and produce supported liquid ionic membranes (SLIMs) that possible for biohydrogen separation.
3.1. Non Porous Polymeric Membranes
- Mass transfer occur in non-porous polymeric membrane is based on solution-diffusion model. The transport rate of H2 is diffusion-based and CO2 is condensable gas and possess solubility-based transport rate [27]. The membrane can be classified H2-selective or CO2-selective polymeric membranes. The H2-selective polymeric membrane is where only H2 is able permeating the membrane and preferred made from glassy polymers. The selectivity of this type of membrane is high when diffusivity of H2 is high and solubility of CO2 is low [28]. Whereas, CO2-selective polymeric membrane allow CO2 to going through it where the less soluble H2 flow as retentate and mostly of these membrane fabricate by rubbery polymers. The selectivity of this membrane is high by increase the solubility of CO2 and decrease the diffusivity of H2.The performance of separation H2/CO2 also can be improved by blend polymeric membranes. The study from Car et al. has proven high selectivity CO2/H2 (10.8) was achieved by using CO2-selective, blend Pebax® MH 1657 with 50 wt.% of polyethylene glycol (PEG) dense membranes than pure Pebax® (9.1 selectivity) [29]. They have tested the performance of this membrane with CO2/N2 and CO2/CH4 gas mixture, but it only enhanced the separation CO2/H2 gas mixture which feasible for hydrogen separation process. However, the selectivity of CO2/H2 when H2/CO2 mixed gas is used is smaller than ideal selectivity in both studies. This phenomenon also occurs in the separation H2/CO2 by using H2-selective membranes. According to study by David et al., the selectivity of H2/CO2 is reduce by 4.2 (ideal selectivity) to 2.7 (measure when 10/90 vol.% H2/CO2 mixed feed gas), by using planar polyimide Matrimid® 5218 membrane [29]. This is because the reduction of available sorption sites on H2-selective membrane for H2 components as these sorption sites has been filled by presence CO2 component, so the transport rate also lowered than pure gas transport rate. There another factor that should be considered in order to select if this membrane applicable to be integrate with biohydrogen production process is elimination of hydrogen sulphide from the gas mixture of fermentation production process. Even though the composition of this gas is very low compare to CO2, but it can reduce the fuel cell performance by contaminant the catalyst of fuel cell in the form of sulfur dioxide [30]. However, there is unavailable research regarding the separation of H2/H2S especially for biohydrogen purification. Hydrogen sulphide separation in biogas processing is a possible way to give a view about the separation of this gas by non-porous polymeric membrane. The study from Harasimowicz et al. has been using polyimide membranes for biogas purification and enrichment by separation of H2S and CO2 [31]. Based on their findings, it is important for further investigate the separation of H2S for biohydrogen separation since the hydrogen was included in the gas mixtures that have been studied. Operating conditions such as feed temperature and pressure are possible impact to mass transfer of gas separation. Theoretically, the higher feed pressure and lower the permeate side pressure leads the higher pressure difference, the greater the flux and higher selectivity can be a can be achieved. Thus, the possible way to do so is by increase the feed partial pressure or lower the pressure on the permeate side. A study by David et al., the high selectivity of H2/CO2 (4.6) at 80/20 vol. % H2/CO2 with H2 partial pressure of 8bar compared to the selectivity of H2/CO2 (3.1) at 20/80 vol. % H2/CO2 with H2 partial pressure of 2bar at the same 10 bar total pressure [32].As mentioned earlier, transport rate of H2-selective glassy polymer membranes and CO2-selective rubbery polymer membranes, are dependent on diffusivity and solubility respectively. The diffusion of gas into membrane is improved by increasing the operating temperature or vice versa. Meanwhile, increasing temperature on reduced the solubility of gas into membranes or vice versa. Bakonyi et al. illustrate high permeability rates of pure H2 and CO2 on H2-selective, polyimide membrane increased as the separation increases from 21-65°C [33]. However, it is essential to determine the effect of temperature on separation of H2/CO2 gas mixture as both component exhibit different gas transport method. This can be demonstrated by study from Car et al., the selectivity of CO2/H2 is decreased as the temperature increase when the 50/50 vol% of CO2/H2 gas mixture is separate by CO2-selective, Pebax®/PEG blend membrane [29]. The study also demonstrated high temperature resulting high CO2 flux, this can be correlated with Arrhenius equation between permeability/flux with temperature [29].Non-porous polymeric membranes can be promising candidates to be coupled with hydrogen producing bioreactors and hence giving the chance for in situ biohydrogen concentration. As the study by Bakonyi et al., they demonstrated that the 18% increase in H2 concentration and 22% decrease in CO2 content can be obtained by using polyimide membrane, where the feed mixed gas of separation unit are generate generate from continuous fermentation process by Escherichia coli (XL1-BLUE) and formate as carbon source [33]. Besides that, non-porous polymeric membrane is also suitable for use in a membrane technology, membrane contractor (MC) since it prevent contaminant of gas with liquid phases.Membrane contactors, MCs is also known as gas-liquid membrane contactor (GLMC), is a device in which the mass transfer between gas to be separated and liquid absorption is occurring through a membrane. Thus, GLMC is generally a combination of membrane absorber (absorption) and membrane desorption (desorption) and operate in a circular mode. The mechanism of gas separation of GLMC system is same with the present absorption-desorption separation system, but the difference is the presence of a membrane between gas and liquid phase which separate them from contact in each unit separation. In the case of H2/CO2 gas mixture, the feed gas mixture will flow in the absorption unit at different direction with input liquid absorbent. During the flow, permeable component, CO2 is going through the polymeric membrane and non-permeable component, pure H2 is flow out the unit as retentate. CO2 that permeable through the membrane is absorbed in absorption liquid. The high concentration CO2 liquid absorbent is then flown in to the desorption unit. In the desorption unit, the CO2 is going through the membrane from the liquid absorbent and the separated CO2 is flow out the system. While, the free CO2 liquid absorbent is pump back to absorption unit to provide a continuous operation.The porous type membrane seem not practical for gas separation in GLMC due to the formation of bubbles in liquid and penetration of liquid into the membrane pore which contaminant the gas product [34]. While the non-porous, polymeric membrane appear more potential to use in membrane contactor since the membrane is possess higher mass transfer coefficient than porous type membrane, able resist high feed pressure and no contaminant take place. Modigell et al. has been demonstrated lab scale GLMC system with Polyvinyltrimethylsilane (PVTMS) membrane with potassium carbonate (K2CO3) as liquid absorbent [35]. High CO2 removal efficiency from H2/CO2 gas mixture can be obtained on their finding. Different liquid absorbent such as monothanolamine (MEA) provide high CO2 separation efficiency (99.7%) than K2CO3 solution (separation efficiency of 57%) that demonstrated in lab scale GLMC that consist of PVTMS membranes [36].The GLMC system also been investigated to couple with biohydrogen production system. A study conducted by Beggel et al. show the about 99% of CO2 separation efficiency from real biogas (H2/CO2 mixture) and high purity hydrogen 90 v/v% can be achieved when integrate membrane contactor with from a photofermentation [37]. The batch separation process is done in their study since the biogas production rate of the photofermerter is too low to operate GLMC. Even though, GLMC have a potential for the separation of hydrogen mixture from biohydrogen production system, but their application get less consideration than supported ionic liquid membranes (SILMs).
3.2. Supported Ionic Liquid Membranes
- Ionic liquids (ILs) are salt-like materials with composition of inorganic anion or organic cation which can be can be adjusted following to the separation task. It is use as replacement of convectional volatile organic solvent that use in liquid membrane technology. This is because ILs has unique properties such as non-volatile characteristic that prevent loss of solution and also fine-tunable properties. Generally, liquid membrane is a non-porous which containing thin layer organic solvent that interposed between to aqueous phases of different compositions [38].ILs is combined with porous polymeric membrane by filled the pores with ILs solvent, act as support/carrier matrixes and transforms porous membrane to non-porous polymeric membranes, known as supported ionic liquid membrane (SILM) [38]. The mechanism of separation in SILMs by the dissolving solute molecules into membrane at the feed/membrane interface and the dissolved species then diffuse through the membrane and desorb at the opposite membrane. ILs also known as room temperature ionic liquids (RTILs), in which the liquid at ambient temperature with no vapour pressure loss by evaporation. The advantage of using SILMs for gas separation is high solubility of different gas components in ionic liquids which make it has potential for separation of H2 and CO2.Cserjési et al. studied the permeability of pure CO2, N2 and H2 and ideal selectivity from binary gas mixture of these components by using four novel VACEM type ionic liquids which integrate with hydrophobic porous PVDF membrane as supporting phases [39]. Based on their study, CO2 has higher permeability than N2 and H2 through the all four VACEM-PVDF membrane and high ideal selectivity of CO2/H2 gas mixtures was obtained. In another research by Cserjési et al., they incorporated twelve unconventional different types of ILs with the similar membrane in order to determine H2, N2, CO2 and CH4 gas separation efficiency [40]. They indicated that not all ILs are suitable for SILMs as they spoiled the PVDF membrane during membrane preparation and high permeability of CO2 are achieved for the rest of applicable ILs compared to other gas.The anion alteration and cation configuration are able change the behaviour of the membranes resulting change of gas separation efficiency. Neves et al. used imidazolium-cation based RTILs with hydrophobic PVDF membrane for separation of H2 from H2/CO2/N2 and CO2 has highest permeabiltity [41]. Their finding showed that the increasing length alkyl chain of RTIL cation and resulting high permeability of pure gas, but it not give any effect to selectivity of gas separation. However, different viscosity anion integrates with imidazolium-based cation give high ideal selectivity. Kanehashi et al. demonstrated increasing permeability of pure gases was obtained by increasing the mass ratio of IL content (51-81 wt %) which incorporated glassy fluorine-containing polyimide (PI) [42]. They stated the formation of IL domain with increase IL mass ratio enhances the gas diffusivity, thus contribute to high permeability.The study for hydrogen separation for mixed gas CO2/H2 specifically from biohydrogen gas by SILMs is currently unavailable. In spite of that, the other mixed gas separation such as CO2/N2 by SILMs can be investigating for future SILMs fabrication considerations. Neves et al. studied the gas separation by using integrated ILs with porous PVDF membrane and their finding show the selectivity CO2/N2 (50 v/v %) gas mixture by is lower than ideal selectivity (112). However, the finding from Gu et al. demonstrated that the selectivity of CO2/N2 (50 v/v %) gas mixture was almost similar with ideal selectivity [43]. They used porous PVDF membrane that filled by supported ion gel as supporting phase which then integrate with IL. The liquid-like gels properties make the selectivity does not depend on diffusivity but from the solubility ratio of ionic liquids, but is not affected when gas mixture exposed at low operating pressure [43].The configuration of SILMs nearly similar to non-porous polymeric membranes, thus the separation principle is based on the diffusivity and solubility of gas mixture which are affected by operating pressure and temperature. Higher gas permeability can be obtained by increasing transmembrane pressure gradient as the driving force for the gas transport is increasing. However, the elevated pressure may contribute to loss of ILs from the pores of supporting material, porous polymeric membrane as well as bring the SILMs to defect or leakages. Therefore, it is important to study the effect of transmembrane pressure to determine pressure limit that membrane able to resist, known as critical displacement pressure which depend on the structure and size of the pores in the support matrix, interface tension of ILs and the contact angle [44]. In order to overcome instability and improved the mechanical durability, many techniques have been applied during fabrication process such as nano-filtration as supporting material [45] and coating the ILs with dense polymer films [46].The study by Gan et al. obtained that the increasing gas permeation rate through the SILMs based nano filtration with increasing feed pressure (3-7 bar), which the pressure way higher than normal SILM (<0.1bar) and the membrane also able maintain stability at high pressure and [45]. However, the gas permeability from Cserjési et al. showed the decrease as the transmembrane pressure increasing (>5 bar) and slightly instability also been observed at pressure greater than 10 bar [40]. The unclear explanation stated in this study that deformation of the size and shape of the supporting polymer membrane is occur at high pressure which reduce the pores volume of separation [40].The operating temperature also affects the permeability and gas separation effiency. Finotella et al. study H2, CO2 and N2 permeability in imidazolium-based room temperature ionic liquids at nearly ambient pressure [47]. Based on their study, high selectivity obtained as the high gas flux generate by high temperature. The explanation of temperature and selectivity effect of SILMs is based on the correlation between temperature and Arrhenius equation. Based on discussion above, non-porous polymeric membrane, membrane contactors and supported liquid ionic membranes have potential to use in biohydrogen separation. However, the research that integrated those membranes with real hydrogen fermentation conditions is limited. Thus, long and extensive research is requiring determining which operation conditions (temperature, pressure) and separation system (single stage, multi-stage, combined different membrane) is feasible for gas separation, where biohydrogen production and separation system can occur together. If high hydrogen purity can be obtained from the biohydrogen integrate system which is feasible to fuel cell technology application.
4. Integrated Biohydrogen and Fuel Cells System
- Fuel cell is an electrochemical energy conversion devices that generating electricity from the conversion of chemical energy. There are various fuel cell technologies that have been develop such as and also their application also varies. The distinct types of fuel cell technologies are including polymer electrolyte membrane fuel cell (PEMFC), alkaline fuel cell (AFC), phosphoric acid fuel cell (PAFC), molten carbonate fuel cell (MCFC), solid oxide fuel cells (SOFC) and direct methanol fuel cell (DMFC). The fuel cell requires fuel and oxygen to produce electricity, heat and water. Hydrogen from biohydrogen separation system is able to utilise as fuel for fuel cell technology. Based on all the fuel cell technologies above, PEMFC has got many attentions due the operation at low temperature and also been demonstrated successfully as portable fuel cell. PEMFC is operate at very low temperatures, below 100°C, use hydrogen proton as mobile ion, and the fuel require is high purity hydrogen and cannot tolerate CO, H2S at concentration (>10ppm) which can poison at low temperature.
4.1. Bioreactor Sizing
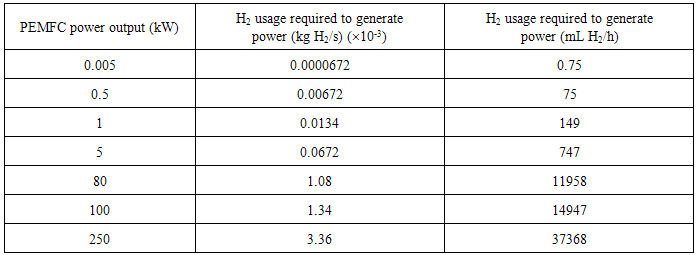
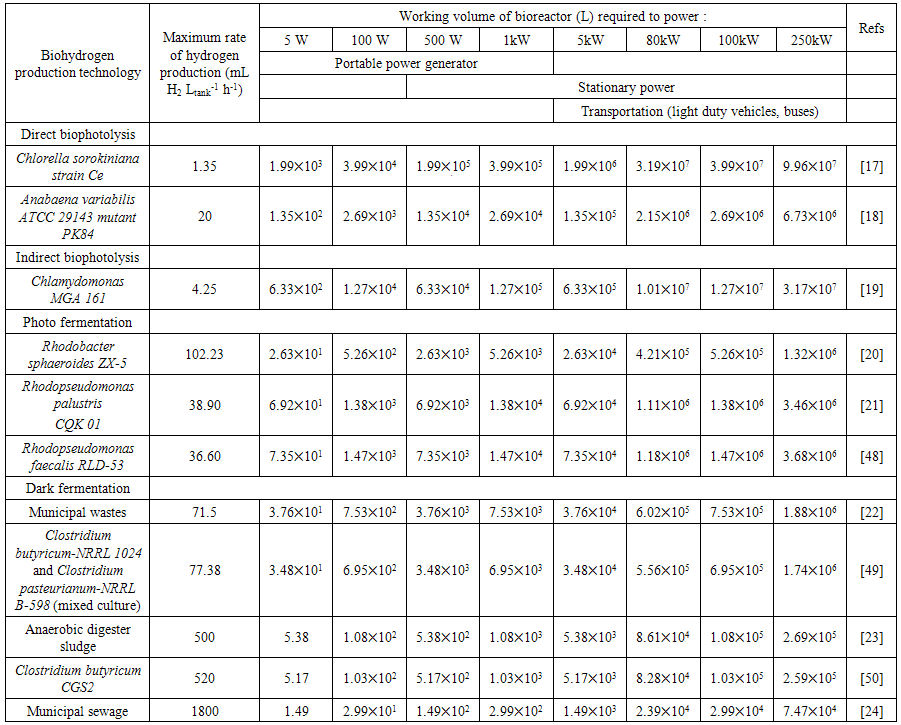
- This analysis is to determining if biohydrogen production technology is sufficient to provide enough hydrogen to power up various fuel cell powers for different applications continuous basis. Besides that, we assume that calculated fuel is containing high purity hydrogen gas from efficiency biohydrogen production integration with hydrogen purification system.Some calculation has been done in order to find hydrogen flow rate require (Table 2) for generate certain power output. These hydrogen flow rate is used to calculate size of bioreactor that produce hydrogen as shown in Table 3.
|
 | Table 3. Sizes of bioreactor required to power the PEMFC |
5. Conclusions
- In a conclusion, dark fermentation would appear the potential candidate to generate hydrogen compare to other biohydrogen production technologies to power the fuel cells at sufficient size and would be applicable for portable fuel cell and stationary power generator. However, more research and development are requiring such as increasing the rates and yields of hydrogen production and optimizing the bioreactor sizing so that hydrogen from biohydrogen production process is able replace non-renewable energy source, fossil fuels and also able to sustain the world power energy supply. Besides that, it is essential to study and extensive research on membrane gas technology in hydrogen separation together with hydrogen fermentation process to determine the possibility of integrated applications.
ACKNOWLEDGEMENTS
- This study was partially supported by UKM – YSD Research Project under code KK-2014-013.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML