-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2015; 5(3A): 1-6
doi:10.5923/c.chemistry.201501.01
Extraction of Cd(II) Ions by Emulsion Liquid Membrane (ELM) Using Aliquat 336 as Carrier
A. L. Ahmad1, M. M. H. Shah Buddin1, 2, B. S. Ooi1
1School of Chemical Engineering, Universiti Sains Malaysia, Engineering Campus, Nibong Tebal, Malaysia
2Fakulti Kejuruteraan Kimia, Universiti Teknologi MARA, Shah Alam, Malaysia
Correspondence to: A. L. Ahmad, School of Chemical Engineering, Universiti Sains Malaysia, Engineering Campus, Nibong Tebal, Malaysia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In this work, the potentialities of vegetable oil to be incorporated as diluent in Emulsion Liquid Membrane (ELM) formulation was explored. Vegetable oil tested in this study are sunflower, corn and palm oil. A compatibility study was conducted to investigate its feasibility to be used as diluent in ELM. It is learned that corn oil showed a promising results to be incorporated in ELM to dissolve Span 80 and Aliquat 336 as surfactant and carrier in the membrane phase, respectively. The analysis showed that Aliquat 336 is highly selective towards Cd(II) as it able to remove almost all Cd(II) ions from the aqueous solution while Span 80 has assisted in the formation of a stable emulsion. The W/O emulsion was prepared using 0.1M NaOH as the internal phase while the membrane phase consist of 3 wt% Span 80 and Aliquat 336, dissolved in corn oil. The influence of stirring speed and extraction time on the solute removal efficiency was observed. The best performance of ELM in extracting Cd(II) ions was recorded at speed of 300 rpm for 15 min. 98.6% of Cd(II) ions were successfully removed from the external aqueous phase at this condition.
Keywords: Emulsion Liquid Membrane, Aliquat 336, Vegetable oil diluent, Cd(II) ions Extraction
Cite this paper: A. L. Ahmad, M. M. H. Shah Buddin, B. S. Ooi, Extraction of Cd(II) Ions by Emulsion Liquid Membrane (ELM) Using Aliquat 336 as Carrier, American Journal of Chemistry, Vol. 5 No. 3A, 2015, pp. 1-6. doi: 10.5923/c.chemistry.201501.01.
Article Outline
1. Introduction
- Rapid industrialization which begun in 1960s has raised many critical issues regarding environmental pollution. One of them is the emission of heavy metal with a great potential to pollute the environment. In fact, Elbagermi et al. [1] reported on the dramatic increasing trend of heavy metal emission since the onset of industrial revolution. In the year 2013 alone, Department of Environment Malaysia reported that a total of 103,994.37 metric tonnes of industrial waste containing heavy metals were generated from various industries in this country [2]. Among the most common heavy metal found in polluted water includes arsenic, copper, cadmium, lead, chromium, nickel, mercury and zinc [3]. Apart from its ability to harm the ecosystem, extensive study carried out by World Health Organization (WHO) has revealed that exposure to these metals will eventually cause deleterious health effects in humans though some of them are nutritionally essential for a healthy life, but in a very small quantity [4]. As for cadmium, it is a toxic metal occurring naturally in the environment and it is considered as a pollutant emanating primarily from industrial and agricultural sources [5]. Due to its high solubility, cadmium can be easily absorbed by living organisms and it can easily enters our food chain [6]. Study conducted by Kadaruddin [7] discovered that as much as 53 rivers in Malaysia’s river system were badly contaminated with cadmium from various sources, mainly manufacturing.Tremendous efforts have been carried out in minimizing the concentration of heavy metal in aqueous media using various physico-chemical treatment [8]. However, Emulsion Liquid Membrane (ELM) which was invented by Li [9] has shown a promising potential in the field of separation. In case of metal removal from aqueous media, ELM was reported to be successfully used in extracting numerous types of heavy metal ions for instance, Cr(VI) [10], Cu(II) [11] and Co(II) [12]. In addition, our previous work on ELM using TOA as carrier has recorded more than 98% of efficiency [13]. In this study, Aliquat 336 was used as the carrier to specifically extract Cd(II) ions. Aliquat 336 is a quaternary ammonium salts which reported to act as anion exchanger at wide range of feed pH [14] and its viscous characteristic has allowed the formation of a stable emulsion against shear. In fact, Aliquat 336 possess surfactant property [15] which is favourable in the production of a stable emulsion.ELM is a developed form of solvent extraction, with the difference that extraction and stripping process occur synchronously in the same unit [16]. In principal, ELM consists of internal (W) and external (W) phase and they were separated by the immiscible organic membrane phase (O) thus, forming W/O/W (water-in-oil-in-water) emulsion system. In this multiple emulsion system, the primary W/O emulsion is prepared first before dispersing it in a second aqueous external phase (W) [17]. Compared to the existing method for heavy metal separation, ELM was reported to be highly selective, having large interfacial area for mass transfer, requires little organic solvent, simple and can be operated continuously [18].In complying with the principle of sustainable development, vegetable oil has been incorporated in various configurations of liquid membrane such as bulk [19] and supported [20], but not in the emulsion type. Commonly, ELM uses petroleum derivatives such as kerosene [21] and hexane [22] as its diluent. These chemicals were known to be non-renewable, highly flammable, difficult to handle, easily volatile and hazardous to living organisms. Also, price inconsistency due to limited supply of petroleum based diluent could affect the total operational cost of ELM. Due to these constraints, the application of petroleum derivatives as diluent in ELM formulation have therefore been called into questions.The main interest of this study is to investigate the potentialities of vegetable oil to be incorporated as diluent in ELM. Their compatibility to be used as diluent in dissolving carrier and surfactant were to be studied. Also, the performance evaluation study of the vegetable oil based ELM in removing Cd(II) ions from the feed phase will be looked at where it involves the study on the effect of stirring speed and extraction time.
2. Materials and Method
2.1. Chemicals
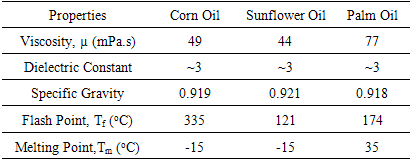
- ELM system consists of 3 main phases; membrane, internal and external phase which were prepared individually. Commercial grade of vegetable oils were used as diluent to dissolve a non-ionic surfactant (Span 80) and carrier (Aliquat 336) purchased from Merck and Sigma Aldrich, respectively. The carrier and surfactant are manufacture grade and used as received. On the other hand, the external phase were prepared using CdCl2 supplied by Sigma Aldrich and the pH of the solution were adjusted using HCl and NaOH solution obtained from Merck. On the other hand, Ammonia solution provided by Merck was used as the internal phase by diluting it to 0.1M.
2.2. Compatibility Study
- In selecting a suitable vegetable oil as an organic diluent, a study to ensure the compatibility of carrier and surfactant dissolved in the oil was carried out. The study was divided into two parts. Firstly, the compatibility of Aliquat 336 in various vegetable oils was investigated. Concept of Simple Liquid-Liquid Extraction was applied in this study, similar to the work carried out by Mohamed et al. [12]. The organic phase was prepared using 10 wt% of Aliquat 336 dissolved in sunflower, corn and palm oil. The study was conducted using equal volume of the organic and feed phase (50 mL), where the latter one containing 150 ppm of CdCl2. The immiscible mixture of organic and feed phase was stirred at 400 rpm at a wide range of feed pH, ranging from 0.8 to 13. The extraction process was carried out for 12 hr at ambient temperature. Once the process ended, the aqueous feed and organic phase were separated using centrifugation at speed of 3000 rpm for 5 min and the concentration of Cd(II) ions in the feed phase was measured. Apart from identifying the most suitable vegetable oil to be used as diluent, another purpose of this study is to identify the best feed pH condition for Cd(II) extraction using Aliquat 336. The second part of this study is to test the compatibility of Span 80 in the emulsion formulation. In forming the membrane phase, 3 wt% Span 80 and Aliquat 336 were dissolved in the selected vegetable oil with the W/O volume ratio of 0.25. The prepared emulsion was placed in a centrifuge bottle and the time for a physical separation of the water and oil phases was recorded [16].
2.3. ELM Preparation
- Preparation of the W/O emulsion started with careful pouring of internal phase into the prepared membrane phase in a beaker. Then, this immiscible mixture was emulsified, assisted by ultrasonic at power of 20W for 12 min. A commercial ultrasonic (USG-150) equipped with titanium horn was used for this purpose.
2.4. ELM for Cd(II) Extraction
- The prepared emulsion was poured into a beaker containing the external phase with 150 ppm of CdCl2. Mechanical agitator with 45o pitch four blade was used to disperse the emulsion in the external phase at ratio 1:5. Once the extraction process completed, the content of the vessel was left for 10 min to allow complete separation of the emulsion and external phase. The external phase was taken out using a syringe for Cd(II) ions concentration measurement and the removal efficiency, E (%) was calculated using the following equation:
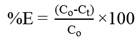 | (1) |
3. Results and Discussion
3.1. Diluent Compatibility Study
3.1.1. Compatibility of Carrier
- Figure 1 displayed the Cd(II) removal efficiency, E (%) data using Aliquat 336 dissolved in three types of vegetable oil. They are corn, sunflower and palm oil and their potentialities to be used in ELM formulation for Cd(II) extraction using Aliquat 336 was investigated. The screening of diluent to be used must be made as the performance of Aliquat 336 for solute extraction is also dependent on the type of diluent used [23]. From the figure, it can be seen that corn oil loaded with Aliquat 336 recorded the best E(%) at acidic medium of feed phase. The results obtained shows that the E(%) has increased following to the order of palm oil< sunflower oil< corn oil.
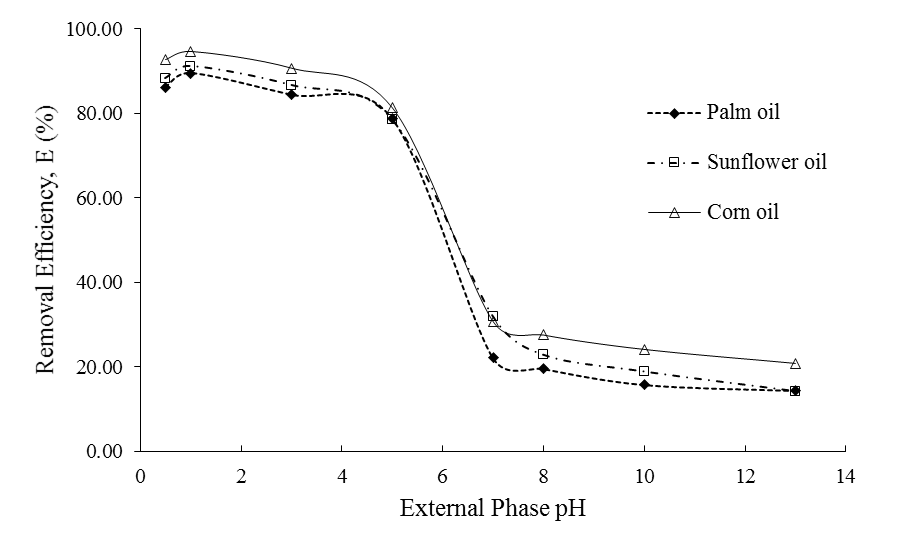 | Figure 1. Cadmium removal efficiency using Aliquat 336 dissolved in various vegetable oils |
|
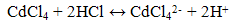 | (2) |
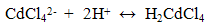 | (3) |
3.1.2. Compatibility of Surfactant
- The subsequent part of this study is to evaluate the stability of the W/O emulsion formed using Span 80 as surfactant in corn oil. The emulsion was formed, shown in Figure 2 and phase separation of the emulsion was observed. It is found that the presence of Span 80 in the emulsion formulation has yield a stable emulsion as it does not show any significant separation of phases for 1 hr, and complete separation of the phases were only observed after 12 hrs. HLB value of 4.3 allows Span 80 to form a stable emulsion using corn oil and Aliquat 336 as diluent and carrier, respectively. In this study, Span 80 has been used to delay the separation of the aqueous and organic phase to ensure that a stable emulsion is formed for a duration of time to conduct the solute extraction process.
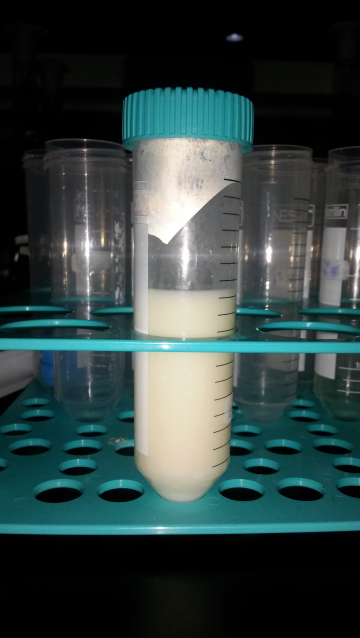 | Figure 2. Prepared W/O emulsion using Span 80 as surfactant |
3.2. Effect of Stirring Speed
- The effect of stirring speed during extraction on Cd(II) removal efficiency, E (%) is presented in Figure 3. It is learned that increasing the speed for dispersion has resulted an abrupt increment in the efficiency. As faster speed is applied during the process, smaller emulsion globules can be formed thus, enhancing the mass transfer area available for Cd(II) extraction. According to the figure, speed around 300 rpm is required to achieve maximum efficiency where 98.2% of Cd(II) ions were removed. In fact, application of sufficient shear has reduced the thickness of membrane layer [32]. This phenomena has further enhanced the transport of solute across the membrane phase as shorter transportation path is created.However, application of unnecessarily faster speed has negatively affect the system. The efficiency has gradually decreased, especially at speed of 500 rpm and beyond. Breakage of the membrane layer is highly possible to be the main cause of such observation. According to Kulkarni and Mahajani [33], membrane breakage could be caused by excessive shear induced by the impeller tip during the extraction process. Likewise, application of fast stirring speed has increased the shear stress which were typically reported to cause membrane film thinning and later, its rupture [34]. This condition was followed by efficiency loss, as reported in Figure 3.
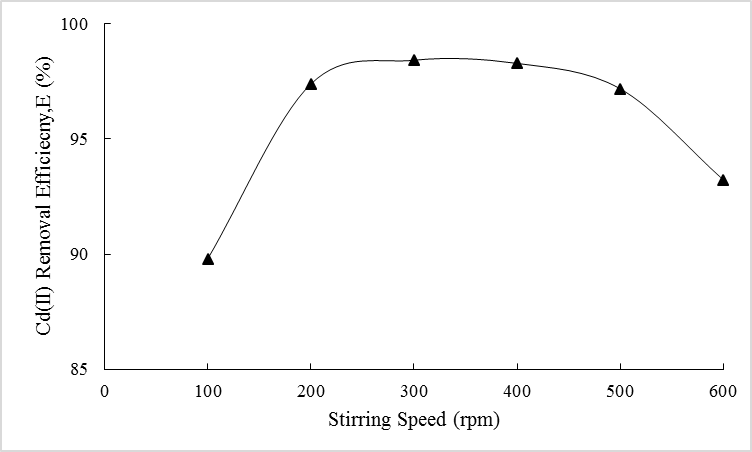 | Figure 3. Effect of stirring speed on Cd(II) removal efficiency |
3.3. Effect of Extraction Time
- Figure 4 showed the dependence of Cd(II) removal efficiency, E (%) on the extraction time. It is learned that the efficiency has increased from 3 to 15 min but it slightly decreased thereafter. At 15 min, 98.6% of Cd(II) ions were successfully removed from the external phase. Low E(%) recorded is caused by the insufficient time (<5 min) available for the Cd(II) ions to form complex with the carrier [35] thus, a great amount of Cd(II) ions still exist in the external phase at the end of extraction process.
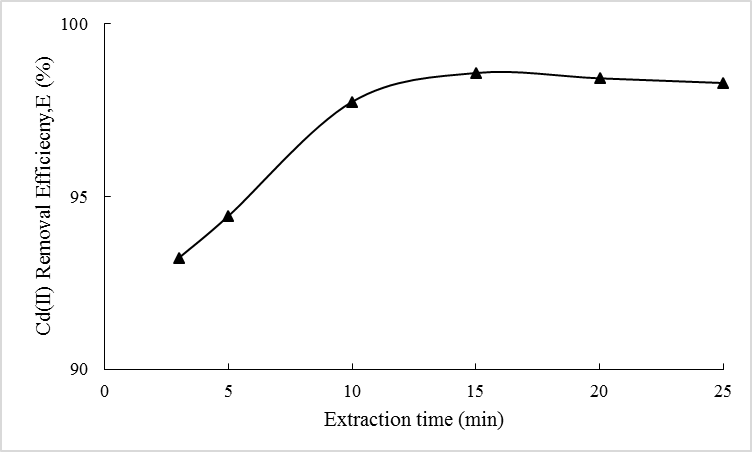 | Figure 4. Effect of extraction time on Cd(II) removal efficiency |
4. Conclusions
- The potentialities of vegetable oil to be incorporated as diluent in ELM was explored. Corn oil showed promising results as diluent to dissolve Aliquat 336 and Span 80 in forming the membrane phase. The study also suggested that acidic condition for the external phase is required to conduct the extraction of Cd(II) ions. The incorporation of Span 80 in the membrane phase has allowed the emulsion to retain its original form, without showing any significant phase separation for at least 1 hr. Also, the influence of two parameters specifically involved during the extraction process were studied. They are stirring speed and extraction time. In overall, it is found that increment of both parameters does not improve the performance of the system. At some extent, a significant drop of the efficiency was recorded. The emulsion and the external phase must be in contact for 15 min and should be stirred at 300 rpm to achieve maximum Cd(II) removal efficiency (98.6%).
ACKNOWLEDGEMENTS
- Financial support by the Membrane Science and Technology Research University Cluster from Universiti Sains Malaysia, Skim Tenaga Pengajar Muda (UiTM) as well as Ministry of Education (SLAB) are greatly acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML