-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2015; 5(3A): 38-43
doi:10.5923/c.ajee.201501.07
Methylene Blue Adsorption onto NaOH Modified Durian Leaf Powder: Isotherm and Kinetic Studies
Zurhana M. Hussin, Norziyanti Talib, Noraini M. Hussin, Megat A. K. M. Hanafiah, Wan K. A. W. M. Khalir
Faculty of Applied Sciences, Universiti Teknologi MARA Pahang, Malaysia
Correspondence to: Zurhana M. Hussin, Faculty of Applied Sciences, Universiti Teknologi MARA Pahang, Malaysia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Durian leaf powder was treated with NaOH to introduce more carboxylate (–COO-) groups in order to improve its performance as an adsorbent. The interaction between chemically treated durian leaf powder (CTDLP) and methylene blue (MB) was examined by using a Fourier Transform Infrared (FTIR) spectrometer. Surface modification of CTDLP was examined by using a Scanning Electron Microscope (SEM). Batch adsorption study was applied to investigate the effects of contact time, initial dye concentration, adsorbent dosage and pH of dye solution on MB adsorption. The experimental data best fitted with Langmuir model (R2 > 0.989) with a maximum monolayer adsorption capacity of 125 mg g−1. The kinetic studies showed good correlation with pseudo-second-order model, suggesting that the MB adsorption involved chemisorption process. This study revealed CDTLP as a potential adsorbent for MB removal from wastewater.
Keywords: Adsorption, Durian leaf powder, Langmuir, Kinetic, Methylene blue
Cite this paper: Zurhana M. Hussin, Norziyanti Talib, Noraini M. Hussin, Megat A. K. M. Hanafiah, Wan K. A. W. M. Khalir, Methylene Blue Adsorption onto NaOH Modified Durian Leaf Powder: Isotherm and Kinetic Studies, American Journal of Environmental Engineering, Vol. 5 No. 3A, 2015, pp. 38-43. doi: 10.5923/c.ajee.201501.07.
Article Outline
1. Introduction
- Dyes are widely applied in many industries such as plastic, dye, textile, dyestuffs, paper, rubber, food, leather, cosmetics, carpet, and printing [1]. The dye effluents from these industries are usually discharged to the environment with or without treatment. Methylene blue (MB) is a basic dyestuff which is extensively used for dyeing cotton, silk and wool [2]. Exposure of excessive amount of MB can lead to adverse effect to human health. Instant contact via inhalation of MB can lead to breathing difficulties, while direct contact with MB may lead to permanent eye injuries, burning sensations, excessive sweating, metal confusion, cyanosis, convulsions, tachycardia and methemoglobinemia disease [3, 4]. The toxicity and carcinogenic characteristics of MB also influence the ecological system by contaminating water with dyes inhibitory to aquatic life [5]. Removal of MB from effluent is difficult because the dye is not easily degradable due to its synthetic and complex aromatic molecular structure [6]. There are several techniques involved in dye removal from effluent such as ion exchange, reverse osmosis, membrane filtration, coagulation, and evaporative recovery techniques [7]. The most common process is adsorption by using activated carbon as adsorbent. This method is highly effective but the running costs are high with the need for regeneration after each sorption cycle [8]. In recent years, many researchers are extensively exploring to develop new alternative and economically adsorbents such as natural-plant based materials, which include sugarcane bagasse [9], Platanus orientalis leaf [10], rice husk [11] and meranti sawdust [12] to remove MB from aqueous solution. Waste tree leaves have attracted many researchers because they contain polyphenolics, plant pigments and protein, which act as active sites for dye binding [10]. In this study durian leaves have been employed as a sorbent to remove the MB from aqueous solution. The effects of contact time, pH, initial MB concentration and temperature on the adsorption process were investigated. Kinetics and isotherm studies related to the process were also performed.
2. Materials and Methods
2.1. Adsorbent Preparation
- The durian leaves, collected from Kampung Temiang, Muar, Malaysia were washed repeatedly with distilled water and dried in an oven at 80°C overnight. The dried leaves were cut into several pieces, ground and sieved to a constant size of 212 μm. Then, the durian lef powder (DLP) was treated with 0.50 M sodium hydroxide (NaOH) solution in a conical flask for 90 min. The samples were filtered and washed thoroughly with distilled water before drying overnight at 80°C. The mixture was stored at room temperature and abbreviated as CTDLP.
2.2. Adsorption Characterization
- The pH of the aqueous slurry was determined by adding 0.10 g of CTDLP into 100 mL of distilled water. The solution was stirred and the final pH was measured after 24 h. The determination of pHzpc of CTDLP was performed by modified addition method [13]. The initial pH of 0.01 M KNO3 (pHi) solutions was adjusted from pH 3 to 9 by adding either 0.10 M HCl or 0.10 M NaOH. Then, 0.10 g of CTDLP was added to 25 mL of 0.01 M KNO3 solution, stirred and the final pH of the solutions was measured after 24 h. The difference between the initial and final pH (pHf – pHi) was plotted against the initial pH (pHi) and pHzpc was determined as the value corresponding to the point where the plot intersected with x-axis at the value where y = 0. The functional groups in CTDLP before and after adsorption with MB were confirmed by scanning the samples at absorption spectrum between 4000 and 400 cm-1 using Fourier Transform Infrared Spectrometer (FT-IR, System 1600, Perkin Elmer, USA). A scanning electron microscope (SEM) (Hitachi S2700, Japan) was used to observe the surface physical morphology on the CTDLP.
2.3. Preparation of MB Solution
- MB used in this study was of commercial purity (C16H18ClN3S.3H2O, FW = 373.90, C.I.52015, kmax = 664 nm). The stock solution (1000 mg L-1) of MB dye was prepared by dissolving required amount of dye in distilled water. The experimental solutions of desired concentration were prepared by diluting stock solution with distilled water and were adjusted to pH 6 by using 0.1 M NaOH and 0.1 M HCl solutions. MB concentration was determined using UV-Vis spectrometer (Shimadzu, Model UV 1601, Japan) at 664 nm of absorbance wavelength (λmax).
2.4. Batch Adsorption Studies
- In general, experiments were carried out by stirring a known weight of CTDLP in 50 mL MB solutions in 100 mL conical flasks and placed in thermostated water bath shaker at room temperature. The effect of pH was performed from 2 to 10. The study of the effect of dosage was carried out from 0.02 g to 0.10 g. Meanwhile, the effect of concentration was carried out at different concentrations (10 to 40 mg L-1) at 30°C and constant shaking rate of 120 rpm. Then, the solution was centrifuged at 3000 rpm for 3 min. The solution was analyzed by using UV-Vis spectrometer (Shimadzu, Model UV 1601, Japan). The amount of MB adsorbed onto adsorbent (qe) and the percentage of removal efficiency (R) were calculated using Equations (1) and (2), respectively.
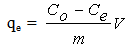 | (1) |
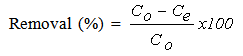 | (2) |
3. Results and Discussion
3.1. Characterization
- FTIR analysis was carried out in order to identify the functional groups in durian leaves. The FTIR spectra are shown in Figure 1. For the DLP, there was a broad peak located at 3436 cm-1 which indicates OH stretching vibration of alcohols, phenols and carboxylic acids as in pectin, cellulose, and lignin [14]. The peak observed at 2851 cm-1 was attributed to the stretching vibration of C–H bond in methyl group. The peak at 1638 cm-1 indicated the presence of a carbonyl group (C=O) in DLP. The peak at 1383 cm-1 indicated the presence of C-N of amide group. The peak around 1060 cm-1 was assigned to C–O–C stretching of cellulose. After the treatment process (CTDLP) and MB loaded, some peaks were shifted or disappeared, and new peaks were also detected. These changes suggested the interactions of MB with the functional groups of the CTDLP during adsorption process.
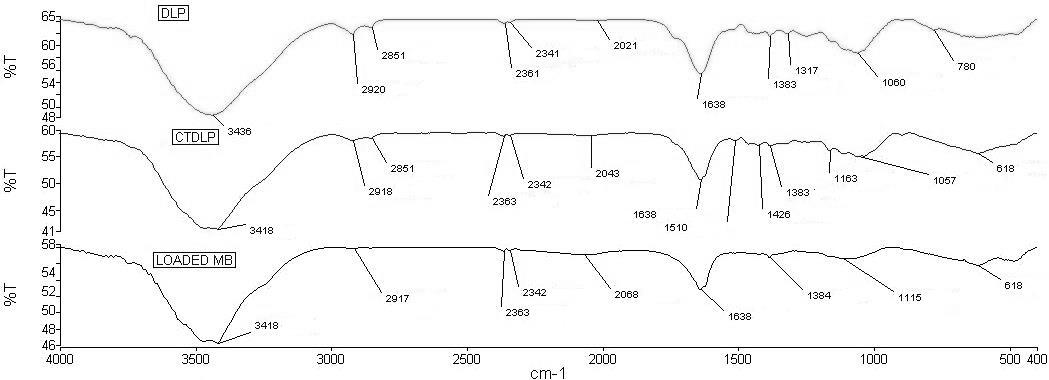 | Figure 1. FTIR spectra of DLP, CTDLP and CTDLP loaded with MB |
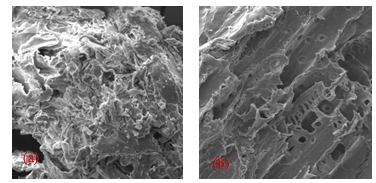 | Figure 2. SEM spectra of adsorbent (a) DLP and (b) CTDLP |
3.2. pHzpc and pHslurry
- pH aqueous slurry was used to confirm whether the adsorbent was acidic or basic [16]. The pHslurry value obtained was 7.1, which indicated that the adsorbent was slightly basic. pHzpc is the point at which the net charge of the adsorbent is zero and it was used to indicate the type of surface active centers and the adsorption ability of the surface [17]. Figure 3 shows that the value of pHzpc was 7.3, which was close to pH of aqueous slurry. If the initial pH of adsorbent is greater than the point of the zero charge (pH > pHzpc). The surface charge of the adsorbent is negative and vice versa [16]. Hence, MB adsorption was considered as more effective at pH > 7.3.
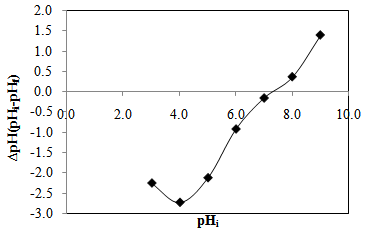 | Figure 3. pHzpc plot of CTDLP |
3.3. Effect of Adsorbent Dosage
- Adsorbent dosage is an important factor that influences an adsorption process since it determines the adsorption capacity of an adsorbent. Figure 4 shows the removal percent of MB increased by increasing the adsorbent dosage. This is due to the availability of more binding sites, which is directly proportional to the mass of adsorbent in the solution [18]. However, increasing the adsorbent dosage decreased adsorption capacity values of MB onto CTDLP because of the decrease of the effective surface area. The adsorbent dosage of 0.04 g was selected to be an optimum dosage for further adsorption studies.
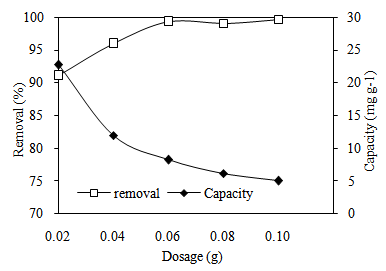 | Figure 4. Effect of adsorbent dosage on adsorption of MB onto CTDLP |
3.4. Effect of pH
- The pH of an aqueous solution is one of the most important parameters in the adsorption process. The rate of adsorption of MB depends on the pH of a solution because H+ and OH- ions tend to compete with MB for adsorption sites [19]. In solution that was considered as acidic, H+ ions caused electrostatic repulsion to occur between cationic dyes by absorbing H+ ions. This caused low adsorption capacity (mg g-1) of dye on the surface of an adsorbent due to competition between these dyes with the H+ ions in the system [20]. Higher pH value resulted in an abundant production of OH- ions. So, the value of adsorption capacity (mg g-1) tends to increase due to the interaction of electrostatic interaction between positively charged of dye with negatively charged CTDLP surface.
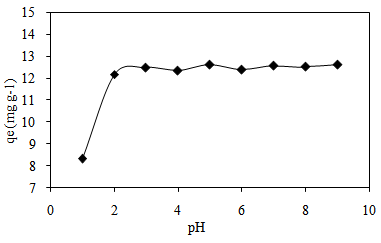 | Figure 5. Effect of pH on adsorption of MB onto CTDLP |
3.5. Effect of Initial MB Concentrations and Contact Time
- The effect of contact time on the adsorption of MB by CTDLP, at three different concentrations (10, 20, and 40 mg L-1) is shown in Figure 6. It is evident from this figure that MB adsorbed increased with increasing contact time. The removal of MB was dependent on the initial concentration. The adsorption equilibrium reached 90 min for MB concentration of 10, 20 and 40 mg L-1. In this study, the MB absorption amount was increased from 23.11 to 44.83 mg g-1 when the concentration of MB increased from 20 mg L-1 to 40 mg L-1. The maximum dye uptakes were 12.35, 24.10 and 46.69 mg g-1 for 10, 20 and 40 mg L-1, respectively. The amount of MB adsorbed was rapid during the first 15 min and then gradually decreased at a slower rate and finally reached the equilibrium point. The rapid initial adsorption occurring within 15 min was related to the vacant surface sites that were available for adsorption of MB during the initial stage [14]. It was followed by slow adsorption rate within the range of 90-120 min before it reached the last phase which is dynamic equilibrium time. Higher adsorption capacity is related to initial dye concentration due to increasing mass transfer driving force and this will lead to the greater interaction between dye and adsorbent [22].
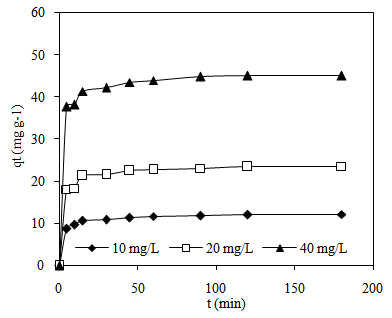 | Figure 6. Effect of concentration and contact time on MB adsorption |
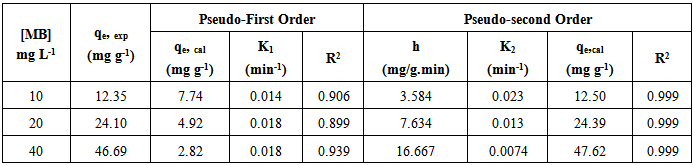
3.6. Adsorption Kinetics
- The efficiency of adsorption process can be measured by applying several kinetic modelling. To evaluate the rate of adsorption of MB, pseudo-first-order and pseudo-second-order kinetic models were applied to the experimental data. The formula of pseudo-first-order equation is shown in Equation (3) [22]:
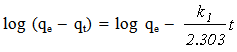 | (3) |
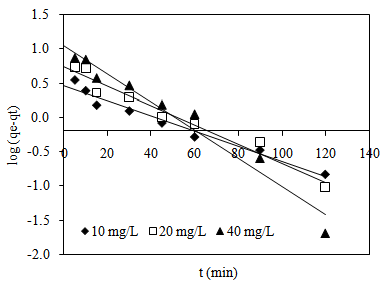 | Figure 7. Pseudo-first-order plots for MB adsorption onto CTDLP |
|
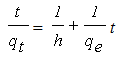 | (4) |
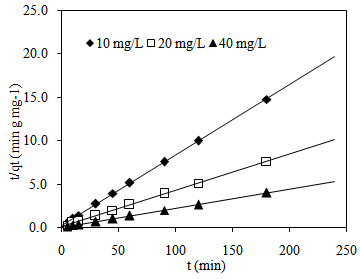 | Figure 8. Pseudo-second-order plots of MB adsorption onto CTDLP |
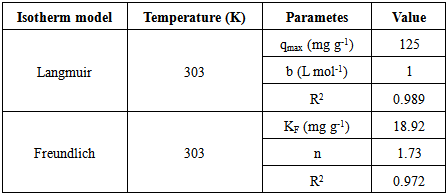
3.7. Adsorption Isotherm
- There are two types of isotherm model that were applied in this study which are Langmuir and Freundlich model. An assumption has been made on Langmuir isotherm that adsorption on a homogenous surface containing site will occur with equal energy that is equally available for adsorption [5]. As Langmuir isotherm is monolayer isotherm, there is no transmigration of adsorbate on the surface plane [24]. The Langmuir equation can be described by the linearized form given by Equation (6).
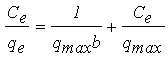 | (6) |
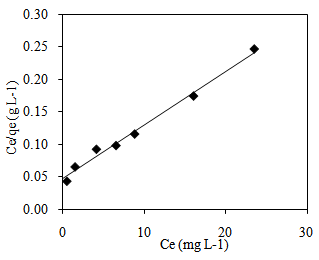 | Figure 9. Langmuir isotherm plot of MB adsorption onto CTDLP |
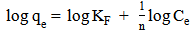 | (7) |
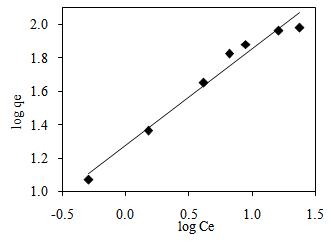 | Figure 10. Freundlich isotherm plot of MB adsorption onto CTDLP |
|
4. Conclusions
- A new potential adsorbent has been prepared by chemical modification of durian leaves powder using NaOH for the removal of MB. The kinetics of MB adsorption on DTLP was best described by pseudo-second-order rate model. The equilibrium data was fitted well with Langmuir isotherm model which showed monolayer adsorption capacity of 125 mg g−1. The above results confirmed the potential of the durian leaves powder as an efficient adsorbent in the MB removal.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML