-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2015; 5(3A): 19-25
doi:10.5923/c.ajee.201501.04
Acid Blue 25 Adsorption onto Phosphoric Acid Treated Rubber Leaf Powder
Khadijah Khalid1, Wan S. W. Ngah2, Megat A. K. M. Hanafiah1, Nur S. A. Malek2, Siti N. M. Khazaai1
1Faculty of Applied Sciences, Universiti Teknologi MARA, Pahang, Malaysia
2School of Chemical Sciences, Universiti Sains Malaysia, Penang, Malaysia
Correspondence to: Khadijah Khalid, Faculty of Applied Sciences, Universiti Teknologi MARA, Pahang, Malaysia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The performance of rubber leaf powder treated with phosphoric acid in the removal of Acid Blue 25 (AB25) from aqueous solution was evaluated. The functional groups on the adsorbent surface were confirmed by Fourier transform infrared (FT-IR) spectroscopy. The effects of several important parameters that can affect adsorption capacities such as pH, agitation period, adsorbent dosage and different initial concentrations of AB25 were studied. In order to describe adsorption isotherm of phosphoric acid treated rubber leaf powder (PARL), the sorption data were analyzed using a linear form of Langmuir and Freundlich equation. The maximum adsorption capacity determined from Langmuir isotherm was 28.09 mg/g at 320 K. Two kinetic models including pseudo-first order and pseudo-second order were used to analyse the AB25 adsorption process, and the result showed that the pseudo-second order fitted well with correlation coefficients greater than 0.99. Therefore, results showed that PARL are favourable adsorbent to be used to remove the AB25 dye.
Keywords: Acid Blue 25, Adsorption, Phosphoric acid, Rubber leaf powder, FT-IR isotherm, Kinetic
Cite this paper: Khadijah Khalid, Wan S. W. Ngah, Megat A. K. M. Hanafiah, Nur S. A. Malek, Siti N. M. Khazaai, Acid Blue 25 Adsorption onto Phosphoric Acid Treated Rubber Leaf Powder, American Journal of Environmental Engineering, Vol. 5 No. 3A, 2015, pp. 19-25. doi: 10.5923/c.ajee.201501.04.
Article Outline
1. Introduction
- The industries are reported to have been releasing about 2 % of dyes into the ecosystem through their effluents of which they are non-biodegradable and being accumulated in the natural water bodies [1]. Textile, paper mills and leather are among the major industries that cause serious environmental problems [2]. The colouration of the water by the presence of dyes even in small concentration is easily detectable and may have an inhibitory effect on the process of photosynthesis [3], thus can affect the aquatic ecosystem. Removing synthetic dyes from the industrial effluent is of great challenge since most dyes are stable to light and heat, high in organic content and have complex aromatic structures. Therefore, various conventional methods have been carried out to remove these soluble coloured contaminants from aqueous solutions. Among all methods, adsorption is known to be the most efficient process to sequester organic compounds from aqueous streams [4], and activated carbon is the most commonly used adsorbent but it is quite expensive [5]. A number of low cost adsorbents have been employed for removal studies of dyes and chemical activation by using phosphoric acid had been attempted to improve pores structure. Rubber trees are widely planted in Malaysia and have become one of the major plantation crops after oil palm. Rubber related industries are now expanding to meet the demands from consumers. However, the vast area of rubber plantation in Malaysia generates an enormous amount of ‘waste’ rubber leaves especially during the dry season (February to March) every year [6]. The application of rubber leaves as an adsorbent for the removal of various heavy metal ions from wastewater have been studied and reported in the literature [6-9]. The physical modification of the rubber leaf powder can provide low surface area, an indicative of low porosity of adsorbent. The average pores diameter suggesting that the treated rubber leaf powder is a mesoporous adsorbent [7]. Despite rubber leaf powder good adsorption capability, further chemical modification could be done to enhance its ability. However no studies have been conducted on phosphoric acid treated rubber leaf powder.In this study, the adsorption of Acid Blue 25 (AB25) onto phosphoric acid treated rubber leaf powder (PARL) was investigated. Experiments were carried out as a function of pH, agitation period, the effect of adsorbent dosage and temperature on adsorption efficiency of AB25. The equilibrium adsorption data of AB25 onto PARL powder were determined using Langmuir and Freundlich equations. Pseudo-first order and pseudo-second order kinetic models were used to determine the adsorption rates.
2. Materials and Method
2.1. Materials
- Dried mature (brownish colour) rubber leaves were obtained from Universiti Teknologi MARA Pahang, Malaysia rubber plantation. All the reagents used throughout the experiments were analytical-reagent grade and utilized without any further purification. Deionised distilled water was used to prepare all solutions.
2.2. Preparation of Phosphoric Acid Treated Rubber Leaf Powder
- Rubber leaves were ground with a mechanical grinder and sieved to obtain a particle size of < 500 μm. Then, 75 mL of 0.5 M H3PO4 solution was added into the flask containing 5 g of the dried rubber leaf powder. The mixture was stirred using a magnetic stirrer at 300 rpm for 1 h, and later repeatedly washed with distilled water. The rubber leaf powder was then filtered using filter paper by gravity filtration. The filtrate was kept for pH testing to ensure that it remained unchanged. The adsorbent was dried in an oven at 80ºC overnight, sieved to obtain particle size approximately < 300 µm before used. The powder was designated as PARL. The structure of PARL before and after AB25 adsorption obtained was characterized by a FT-IR (Perkin-Elmer System 2000 Model, USA) spectrometer.
2.3. Batch Adsorption Experiments
- A stock solution of 500 mg/L of AB25 dye solution was freshly prepared by weighing 0.25 g and dissolved in 500 mL volumetric flask and finally top up with deionised distilled water until the mark. The absorbance of the standard AB25 solution was determined using an ultraviolet-visible spectrophotometer (Perkin Elmer Lambda 35, USA) at a maximum wavelength of 601 nm.The effect of initial pH of the solution on the adsorption of AB25 onto PARL was performed in the pH range of 2-10. Five sets of 100 ml AB25 dye solutions with a concentration of 20 mg/L were prepared. NaOH and HCl solution with appropriate concentrations were used to adjust the pH to ensure that there was no change in the concentration of the solution. Then, 0.10 g of PARL was transferred into the solution and left for shaking by using Heidolph Unimax 1010 shaker for 2 h at the particular pH. The pH that gave the highest adsorption capacity was used for the rest of the study. The effect of agitation time was also studied to determine the optimum condition for the adsorption of AB25 by varying the agitation time from 30-180 min at pH 2. The effect of adsorbent dosage was investigated by varying the initial mass of the adsorbent in the range of 0.01-0.1 g. The percentage removal of AB25 was calculated by the following equation:
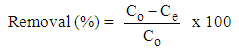 | (1) |
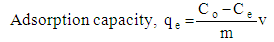 | (2) |
3. Results and Discussions
3.1. FT-IR Analysis
- A comparison of FT-IR spectra of unmodified rubber leaf powder, PARL before and after AB25 adsorption is shown in Figure 1. From Figure 1(a) and Figure 1(b), it was found that the FT-IR spectra for all the samples do not display many variations. The broad and high band ranging from 3000 to 3600 cm-1 indicated the overlapping of Si–OH (silanol), R–OH (hydroxyl) and –NH2 (amine) stretching vibrations [9]. The sharp peaks observed at 2922 and 2850 cm-1 can be assigned to asymmetric and symmetric –CH2 groups. The presence of carboxylic acid (RCOOH) is shown by the peaks located at 1732 and 2400-3600 cm-1.
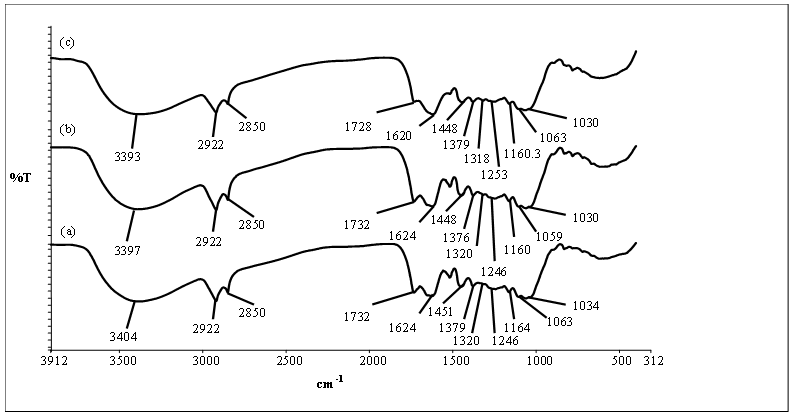 | Figure 1. FT-IR spectra of (a) unmodified rubber leaf powder, (b) PARL before AB25 adsorption and (c) PARL after AB25 adsorption |
3.2. Effect of Initial pH
- Effect of pH on adsorption of AB25 dye onto PARL is shown in Figure 2. From Figure 2, pH 2.0 was found to be the optimum pH of adsorption for AB25 dye onto PARL. The adsorption capacity decreased with the increase in pH. PARL had maximum dye removal at 99.34 % at pH 2 and dropped to 7.68 % at pH 10. As the pH increased, the number of positively charged adsorbent surface would be reduced. This phenomenon would not favour the adsorption of AB25 due to the reduction in the electrostatic attractions [2] and is recognized as the important role played by solution pH towards the dye removal onto PARL.
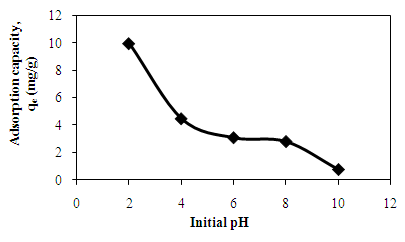 | Figure 2. Effect of initial pH on the adsorption of AB25 onto PARL |
3.3. Effect of Agitation Time and Initial Dye Concentration
- Effect of agitation time on adsorption of AB25 dye onto PARL is shown in Figure 3. The adsorption capacity increased with the increased in agitation time until equilibrium or optimum time was achieved. From the graph of adsorption capacity against agitation time, after the optimum time was reached, further agitation time did not increase the adsorption capacity. This condition showed that the process or system is in an equilibrium state. Hence, the optimum agitation time for the adsorption of AB25 dye onto PARL is 120 min.
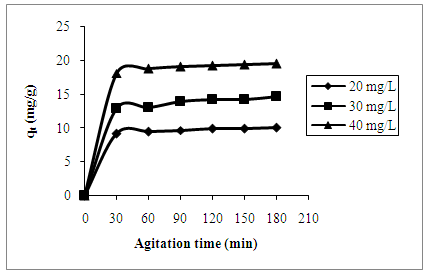 | Figure 3. Effect of agitation time on the adsorption of AB25 onto PARL |
3.4. Effect of Adsorbent Dosage
- The amount of dye removed by adsorption on PARL is presented in Figure 4. The dosage of adsorbent varied from 0.01 to 0.10 g at fixed pH and adsorbate concentration. From Figure 4, it was found that the percentage removal of the dye had increased from 69.5 to 99.4 % with the increased in adsorbent dosages. There was a decrease in amount of AB25 adsorbed with increased of the mass of adsorbents. According to Rajeev et al. (2014) [11], as the adsorbent dosage increases, the adsorbent sites available for the molecules also increases and consequently better adsorption takes place. Hence, the increase in the amount adsorbed with the dosage can be attributed to the increase in surface area and availability of more adsorption sites. From the graph, the amount of AB25 adsorbed decreased from 69.48 to 9.94 mg/g as the dosage was increased from 0.01 to 0.10 g using 50 mL of 20 mg/L AB25 in the solution. This condition may be due to the overlapping of adsorption sites as a result of overcrowding of adsorbents particles [12].
 | Figure 4. Effect of adsorbent dosage on the adsorption of AB25 onto PARL |
3.5. Adsorption Equilibrium Isotherms
- An adsorption isotherm is an expression that shows the relationship between the amounts of adsorbate adsorbed per unit weight of adsorbent (qe, mg/g) and the concentration of adsorbate in the bulk solution (Ce, mg/L) under equilibrium conditions. The general isotherm plot as in Figure 5 shows the adsorption capacity is increased as the temperature increased.
 | Figure 5. Adsorption isotherm of AB25 dye solution onto PARL at 300, 310 and 320 K |
 | (3) |
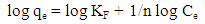 | (4) |
|
3.6. Adsorption Thermodynamics
- In order to determine the spontaneity of a process of adsorption, three main thermodynamic parameters were used, namely enthalpy change (∆Hº), entropy change (∆Sº) and Gibbs free energy change (∆Gº) to test the experimental data obtained. All the thermodynamics parameters, such as ∆Hº, ∆Sº and ∆ Gº can be calculated from the following equations [8]:
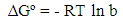 | (5) |
 | (6) |
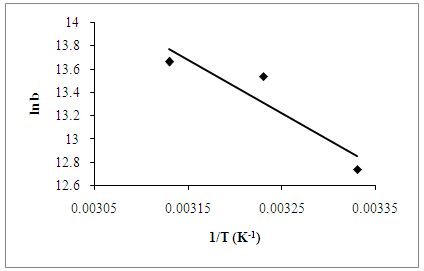 | Figure 6. Van’t Hoff plot of AB25 adsorption onto PARL |
|
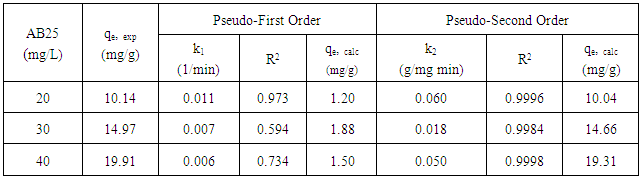
3.7. Adsorption Kinetics
- Pseudo-first and pseudo-second model are used to explain the mechanism of adsorption. Kinetic adsorption data were first treated with pseudo-first order kinetic model. The linear form of pseudo-first order rate equation is expressed as Equation 7 [15]:
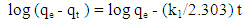 | (7) |
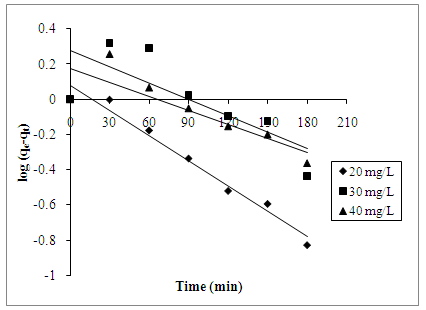 | Figure 7. Pseudo-first order kinetic reaction of AB25 adsorption onto PARL at 20, 30, and 40 mg/L |
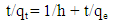 | (8) |
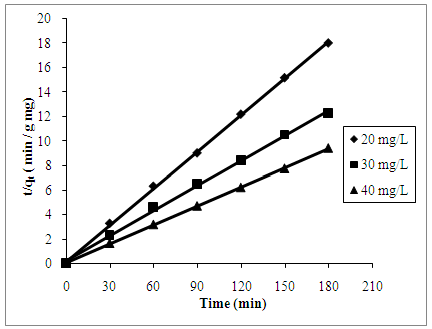 | Figure 8. Pseudo-second order kinetic reaction of AB25 adsorption onto PARL at 20, 30 and 40 mg/L |
|
4. Conclusions
- PARL showed good adsorption capacity of AB25 dye solution. Adsorption capacity was affected by low pH and higher adsorbent dosage as well as increased in concentration and contact time. AB25 adsorption onto PARL obeyed the pseudo-second order kinetic model while equilibrium data presented a better fit to the Langmuir, isotherm model. The FT-IR analysis revealed -OH, -NH2, -COOH, C=C and -COO- as the major functional groups that act as adsorption sites for AB25 dye solution. Hence, it can be concluded that PARL adsorbents is effective for the uptake of AB25 dye.
ACKNOWLEDGEMENTS
- The authors wish to extend their appreciation to the staff of the School of Chemical Sciences, Universiti Sains Malaysia, Penang, Malaysia for their assistance in this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML