-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2015; 5(3A): 13-18
doi:10.5923/c.ajee.201501.03
Removal of Acid Violet 7 Using Chitosan-GLA as the Adsorbent
Megat A. K. M. Hanafiah1, Wan S. W. Ngah2, Nurfarahin M. Safuan2, Wan K. A. W. M. Khalir1, Zurhana M. Hussin1
1Faculty of Applied Sciences, Universiti Teknologi MARA Pahang, Malaysia
2School of Chemical Sciences, Universiti Sains Malaysia, Malaysia
Correspondence to: Zurhana M. Hussin, Faculty of Applied Sciences, Universiti Teknologi MARA Pahang, Malaysia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The removal of Acid Violet 7 (AV 7) by adsorption onto chitosan-glutaraldehyde (chitosan-GLA) beads was investigated under batch adsorption method. Existence of amino (-NH2) and hydroxyl (-OH) groups as the active sites on chitosan-GLA beads was confirmed by Fourier Transform Infra-Red (FTIR) spectrometer. The optimum pH and contact time for good performance of AV 7 adsorption were pH 4 and 120 min, respectively. Adsorption kinetics was best described by the pseudo-second-order kinetic model, and the adsorption isotherm study showed a better fit of equilibrium data by Langmuir isotherm model. The maximum adsorption capacity (qmax) obtained was 172.41 mg/g at 323 K.
Keywords: Acid Violet 7, Adsorption, Chitosan-GLA beads, Isotherm, Kinetics
Cite this paper: Megat A. K. M. Hanafiah, Wan S. W. Ngah, Nurfarahin M. Safuan, Wan K. A. W. M. Khalir, Zurhana M. Hussin, Removal of Acid Violet 7 Using Chitosan-GLA as the Adsorbent, American Journal of Environmental Engineering, Vol. 5 No. 3A, 2015, pp. 13-18. doi: 10.5923/c.ajee.201501.03.
Article Outline
1. Introduction
- Nowadays, there has been an overwhelming growth in the production and use of synthetic chemicals. Most of these chemicals have been found to contaminate water supplies over the years. The presence of dyes in water can cause some changes to the physical, chemical and biological properties of water thus, can harm aquatic life and human health. Modern legislation is often compelled to enforce more restrictive regulations for effluent discharges due to the rise of public concern on health. As a result, various treatment systems have been accomplished to eliminate this type of pollutant from aqueous solutions.The practice of using conventional materials for dye removal in wastewater treatment has become a major concern for researchers in line with their environmental impact. The use of toxic materials in removal process contributed to the secondary pollution problems especially from their by-products. The natural biopolymers of chitin and chitosan have been widely used and become one of the developing methods for removing organic pollutants [1]. Chitin is the second most important polymer in nature after cellulose and can be extracted from crustacean shells such as prawns, crabs, fungi, and insects [2].Chitosan can be considered as natural biopolymer due to the properties of non-toxicity, biodegradability, polyelectrolicity and possibility for transformation, which leads to a number of applications in removing dye pollutants [3].Chitosan is a type of natural polyaminosaccharide synthesized from deacetylation of chitin, consisting predominantly of linear chains of β-(1→4)-actoamido-2-deoxy -D-glucose. Chitosan can be used as an adsorbent to remove heavy metals and dyes due to the presence of amino (-NH2) and hydroxyl groups (-OH), which can act as the active sites [4]. Physical or chemical modification can be performed to modify hydroxyl and amino groups of chitosan. Physical modification improved the sorption properties, gel formation and implicated an expansion of the porous network. Chemical modification, otherwise, improved the sorption properties of chitosan while hindering the dissolution of chitosan in strong acids [5]. It also enhances the mechanical strength of chitosan and enhances superficial area of the beads.Cross-linking is one of the most common chemical modifications used to strengthen the stability of chitosan in acidic solutions. It can change the crystalline nature of chitosan and improves the resistance of chitosan over acid, alkali and chemicals, thus increases the sorption abilities of chitosan. Some of the cross-linking agents usually used are glutaraldehyde (GLA), epichlorohydrine (ECH) and ethylene glycol diglycidyl ether (EGDE) [6]. Figure 1 shows the chemical structure of chitosan-GLA.
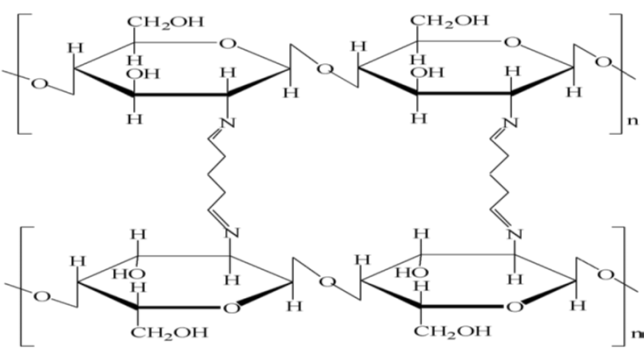 | Figure 1. Chemical structure of chitosan-GLA [3] |
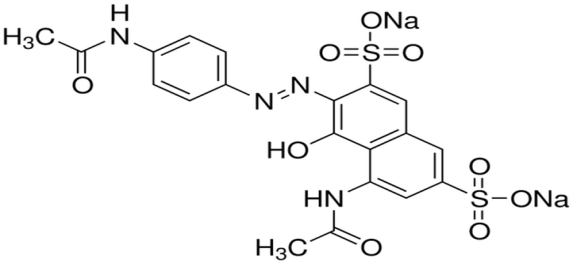 | Figure 2. Chemical structure of AV 7 |
2. Materials and Methods
2.1. Adsorbent Materials and Chemicals
- A sample of chitosan flakes (75-85% deacetylated) was obtained from Chito-Chem (M) Sdn. Bhd., Malaysia. All chemicals used were of analytical grade and used without any further purification. Distilled water was used throughout the adsorption experiments.
2.2. Preparation of Chitosan Beads and Chitosan-GLA Beads
- Chitosan beads were prepared by dissolving 2.0 g of chitosan flakes with 80 mL (5% v/v) acetic acid. The mixture was left overnight and became clear yellow gel. Then, the mixture was added dropwise into 500 mL (0.50 M) NaOH solution under continuous stirring and the beads were allowed to immerse for 15 min. The chitosan beads were rinsed thoroughly with distilled water for several times and filtered. The chitosan beads were air dried before finally ground with a mortar and sieved to obtain an adsorbent size of <250 µm. The method of preparing chitosan-GLA beads was similar to the preparation of chitosan beads except before the chitosan beads were air dried, the chitosan beads were stirred in 150 mL (0.025 M) GLA solution for overnight. The beads were then ground and sieved to <250 µm of adsorbent size.
2.3. Functional Group Identification
- The functional groups in chitosan beads, chitosan-GLA beads before and after adsorption were detected by using a Fourier transform infra-red (FTIR) spectrometer (Perkin Elmer 1600, USA). The spectra were recorded in the wavenumber range from 4000 to 400 cm-1 by potassium bromide (KBr) pellet technique.
2.4. Batch Adsorption Studies
- A stock solution of 500 mg/L AV 7 was prepared each time adsorption experiment was conducted. A mass of 0.25 g AV 7 was dissolved in a 250 mL beaker with distilled water before being transferred into a 500 mL volumetric flask. The stock solution was then diluted to 20 mg/L (unless otherwise stated). In general, adsorption experiments were done by adding 0.01 g of chitosan-GLA beads into 50 mL AV 7 solutions in 100 mL beakers. The mixture was agitated using Memmert water bath shaker (Model WNB 14, Germany) at specific time and room temperature (298 K). After adsorption, the solutions were filtered and filtrates were analysed by using a UV-VIS spectrophotometers (PerkinElmer Lambda 35, USA). The effect of initial pH of the solution on adsorption of AV 7 was carried out in the pH range of 2-12. The initial pH of AV 7 solution was adjusted by adding drops of HCl or NaOH solutions. The pH dependence study was accomplished at agitation speed of 120 stroke/min. The effect of adsorbent dosage was evaluated by varying the initial weight of chitosan-GLA beads in the range of 0.01-0.10 g. The percentage removal (%) of AV 7 was calculated by using equation (1):
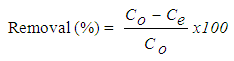 | (1) |
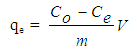 | (2) |
3. Results and Discussion
3.1. Effect of Initial pH
- The effect of initial pH of AV 7 was studied to determine the optimum pH for maximum removal of AV 7. The effect of pH can be explained by the surface charge of chitosan-GLA beads and ionic species of AV 7 in an aqueous solution. Tsai et al. [7] reported that dye adsorption increased with increasing the electrostatic attractions between positively charged of adsorption site and negatively charged of anionic dye. Figure 3 shows the effect of different initial pH on the adsorption of AV 7 onto chitosan-GLA.
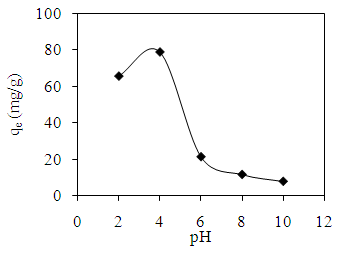 | Figure 3. Effect of initial pH on the adsorption of AV 7 solution onto GLA-chitosan |
3.2. Effect of Adsorbent Dosage
- The effect of adsorbent dosage is shown in Figure 4. The percentage removal of AV 7 increased with increasing the dosage of chitosan-GLA beads due to the greater availability of binding sites. The highest percentage of AV 7 removal was 82.59% at 0.10 g chitosan-GLA beads. However, the amount of AV 7 adsorbed (qe) decreased as the amount of chitosan-GLA beads increased. These results showed that the smaller dosage of adsorbent implies the higher amount of AV 7 in contact with per unit weight of chitosan-GLA beads. The effective contact between an adsorbate and adsorbent surface would increase the amount of adsorbate adsorbed [8]. This trend of observation was found to be similar to most adsorption study using chitosan [9].
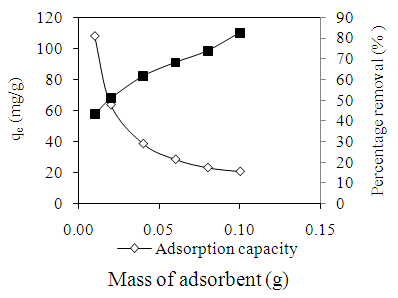 | Figure 4. Effect of adsorbent dosage on the adsorption of AV 7 solution |
3.3. Effect of Agitation Time
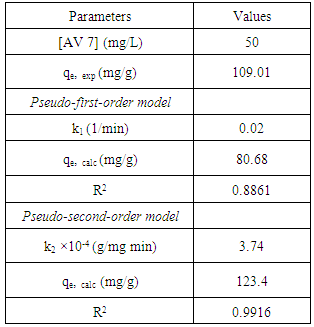
- The amount of AV 7 adsorbed was found to be increased with time before attaining equilibrium as shown in Figure 5. In general, the rate of adsorption consists of two phases; an initial rapid phase and a slow second phase until equilibrium was accomplished. The first phase occurred instantaneously which is related to the exterior surface adsorption. The second phase is the steady stage before adsorption reaches equilibrium. It took about 120 min to attain equilibrium for AV 7 concentration of 50 mg/L. The amount of AV 7 adsorbed at equilibrium was 109.01 mg/g.
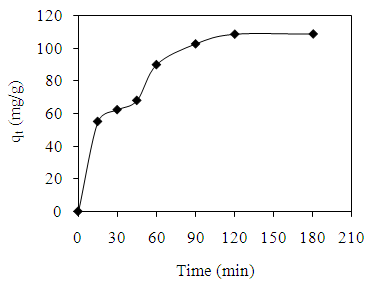 | Figure 5. Effect of agitation time on the adsorption of AV 7 solution |
3.4. Adsorption Kinetics
- In order to investigate the rate determining steps involved in the adsorption process, pseudo-first-order and pseudo-second-order models were used to test the experimental data. It is a typical practice to classify the correlation coefficient, (R2), of an appropriate model and to analyze the adsorption capacity for conformity between the experimental results and the model’s predicted value. The pseudo–first -order kinetic model is based on the assumption that the adsorption rate is proportional to the number of free adsorption sites. This kinetic model is similar to the diffusion expression attained for the case of diffusion through a boundary liquid film [10]. The pseudo-first-order is represented by equation (3) [11]:
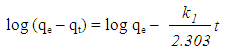 | (3) |
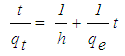 | (4) |
|
3.5. Adsorption Isotherms
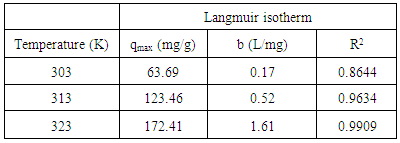
- An isotherm study represents the relationship between the amount of adsorbate adsorbed per unit weight of adsorbent (qe, mg/g) and the concentration of adsorbate in the bulk solution (Ce, mg/L) at a given temperature under equilibrium conditions. An adsorption isotherm is very important in providing information on adsorption mechanisms, surface properties and affinity of an adsorbent for adsorbates [13]. Langmuir and Freundlich isotherms are the most common isotherm models applied in solid-liquid adsorption system. The Langmuir model assumed that the surface containing the adsorbing sites is perfectly flat plane with no corrugations (homogeneous), and also when an adsorbate molecule occupies a site, no further adsorption can occur at this site. The Langmuir equation can be expressed by equation (5):
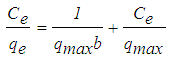 | (5) |
|
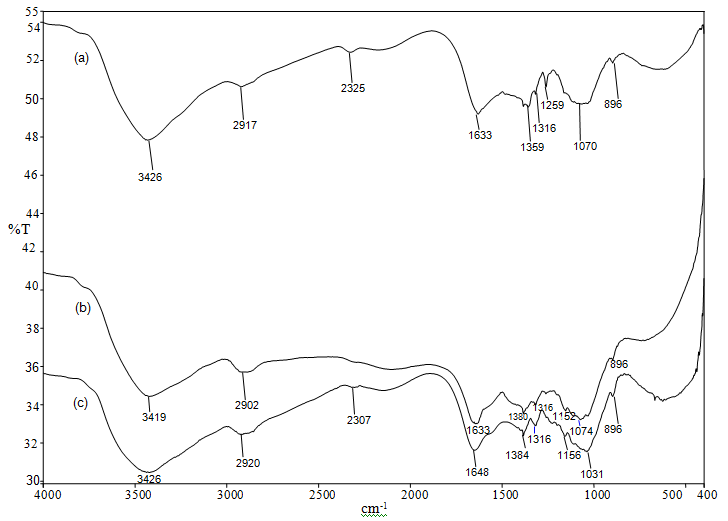 | Figure 6. FTIR spectra of (a) chitosan beads, (b) chitosan-GLA beads and (c) chitosan-GLA beads after AV 7 adsorption |
4. Conclusions
- In this experiment, the adsorption behaviour of chitosan-GLA beads for AV 7 was evaluated. The maximum adsorption capacities (qmax) obtained from Langmuir isotherm model were 63.69, 123.46 and 172.41 (mg/g) at temperatures 303, 313 and 323 K respectively. The qmax value increased with increasing the temperature, thus suggesting that the adsorption of AV 7 onto chitosan-GLA beads was endothermic in nature. The FTIR spectrum of chitosan beads after AV 7 adsorption showed shifting of peaks at 3426 and 1633 cm-1, confirming that hydroxyl and amino groups were the active sites for adsorbing AV 7. FTIR analysis also suggested that the possible mechanism was electrostatic interaction between protonated amino and the hydroxyl group with AV 7. This possible mechanism was also supported by the result from the effect of pH. At pH 4, the amino group were protonated and able to adsorb AV 7 via electrostatic attraction. Based on the results, it can be concluded that the chitosan-GLA bead is an effective adsorbent for the removal of AV 7 from aqueous solutions since a high adsorption capacity was achieved and the mechanical strength of the adsorbent was improved by this modification.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML